What makes TRIDENT different
Diabetic kidney disease is common, heterogeneous, and biologically complex. Many studies describe risk factors and outcomes, but few are designed to directly interrogate disease mechanisms in the human kidney. TRIDENT was built to address this gap.
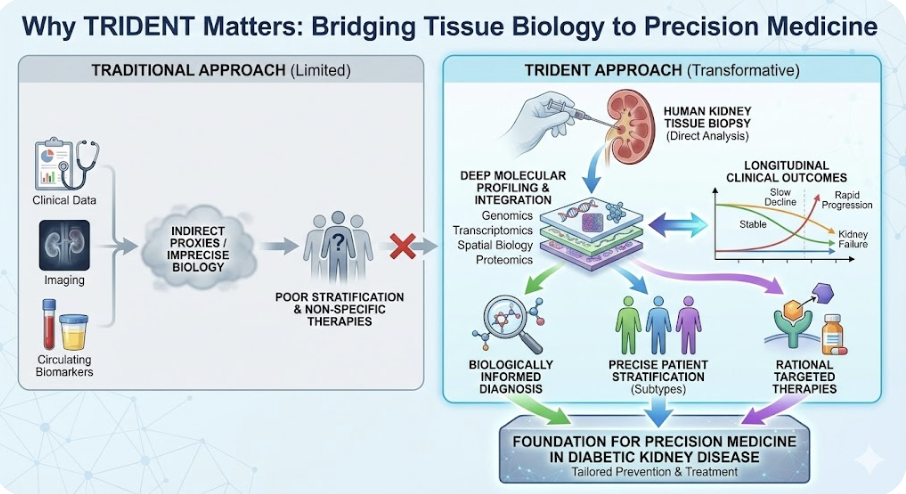
Human kidney tissue at the center
Most kidney disease studies rely on clinical variables, imaging, or circulating biomarkers as proxies for kidney biology. TRIDENT is fundamentally different. It is anchored in clinically indicated human kidney biopsies, allowing direct analysis of the target organ in which disease occurs.
By studying kidney tissue rather than inferring biology from peripheral signals alone, TRIDENT enables precise investigation of the cellular and molecular pathways that drive diabetic kidney disease.
Molecular depth linked to longitudinal outcomes
TRIDENT integrates histopathology with next-generation molecular profiling, including genomics, transcriptomics, spatial biology, proteomics, and biomarker analysis. These data are not analyzed in isolation. They are explicitly linked to longitudinal clinical outcomes, including kidney function trajectories and progression to kidney failure.
This design allows TRIDENT to distinguish molecular features associated with rapid progression from those linked to stable disease, moving beyond cross-sectional description toward outcome-relevant biology.
Biologically informed disease stratification
Diabetic kidney disease is not a single entity. TRIDENT is designed to identify biologically distinct disease subtypes that are not captured by traditional clinical classification.
By integrating tissue-level molecular signatures with histologic patterns and clinical follow-up, TRIDENT supports biologically grounded patient stratification, enabling more precise risk prediction and therapeutic targeting.
Built for translation, not just discovery
TRIDENT was designed from the outset as a translational platform, not solely a discovery cohort. The study infrastructure supports:
-
Identification of tissue-informed diagnostic and prognostic biomarkers
-
Validation of non-invasive biomarkers in blood and urine
-
Discovery of molecular pathways suitable for therapeutic targeting
-
Generation of data to inform future clinical trial design
This translational focus distinguishes TRIDENT from descriptive registries and retrospective cohorts.
A scalable, collaborative consortium
TRIDENT operates as a coordinated, multi-center public–private consortium spanning 16 academic medical centers across North America, with active industry partnerships. This structure enables standardized protocols, centralized data analysis, and sufficient scale to study disease heterogeneity that cannot be captured at a single site.
The consortium model also accelerates translation by aligning academic discovery with therapeutic and biomarker development efforts.
A platform, not a single study
TRIDENT is intentionally structured as an evolving platform.
-
TRIDENT 1.0 focuses on defining disease mechanisms and outcome-associated molecular signatures.
-
TRIDENT 2.0 extends this framework to examine how disease-modifying therapies alter kidney molecular pathways, leveraging advanced spatial technologies and archived clinical tissue.
This platform approach allows TRIDENT to adapt to emerging technologies and therapeutic questions while preserving continuity of data and cohorts.
Why this matters
By directly linking human kidney tissue biology to longitudinal clinical outcomes at scale, TRIDENT provides capabilities that are not achievable through clinical data or biomarkers alone. This approach establishes a foundation for precision medicine in diabetic kidney disease, enabling biologically informed diagnosis, patient stratification, and the rational development of targeted therapies.
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser. | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team.
