Data Management Plan
At the start of the study the research team should determine what data management tool will be used to support the trial. The majority of studies conducted at Penn will be done so using an electronic data capture (EDC) system for housing study case report forms (CRFs). For externally sponsored trials, typically, the sponsor will dictate what system should be used. For investigator-initiated Penn trials there are variety of options that may be used and are detailed below. While paper remains an option, it is not recommended as it does not lend itself to any remote work capabilities and makes submissions to regulatory authorities, off-site monitoring more difficult.
Data Management for Clinical Research eLearning Modules
The Office of Clinical Research’s eLearning series on Clinical Data Management (CDM) is a four-module training that provides a comprehensive foundation for managing research data in clinical studies and trials. Whether you're new to data management or seeking a refresher, each module offers practical guidance, interactive knowledge checks, and links for resources.
Module 1 | Clinical Data Management - OCR
Module 2 | Proper Data Planning
Module 4 | Post-eCRF Build Activities
Data Management with respect to clinical research studies dictates the data points which will be collected as part of the trial and analyzed. All data points collected should correspond to elements within the trial. At the start of a trial a clinical trial database should be decided upon. The following primary choices exist at Penn – REDCap, Veeva CDMS/EDC and Penn CRMS.
The four main resources for data management services are:
For studies where data management is being performed by individual research teams, there are a series of documents to assist with overall data management.
Below is a list of Electronic Data Capture (EDC) systems:
-
REDCap (Penn Medicine): Research Electronic Data Capture – for studies not under an IND, IDE or abbreviated IDE
-
Veeva EDC/ CDMS - HIPAA and Part 11 compliant
-
Penn CRMS – HIPAA and Part 11 Compliant
The following table provides a comparison of the three primary options: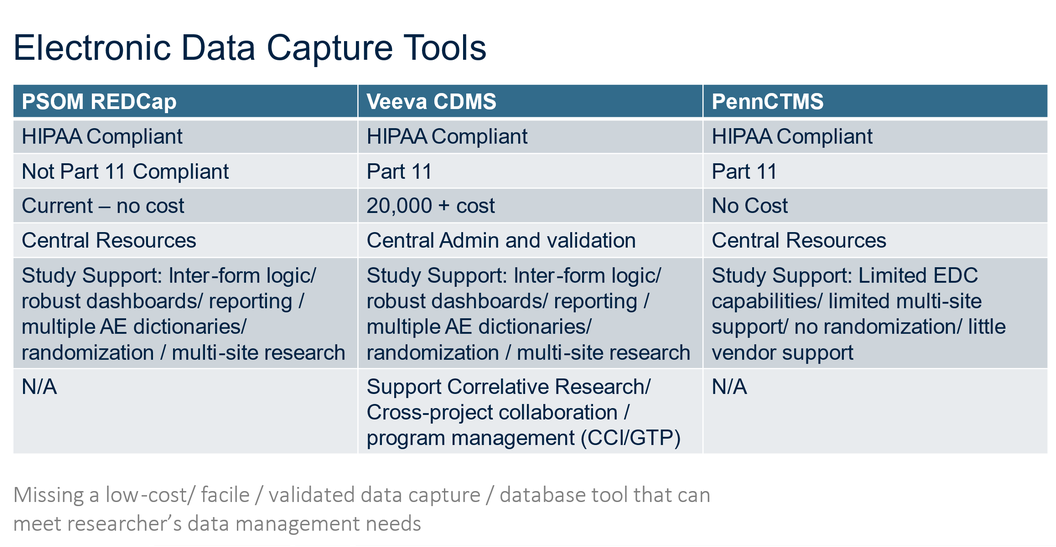

Note: For statistical software and packages consult with IBI or the CCEB BAC.
What is 21 CFR Part 11 and does it Apply to my Study?
21 CFR Part 11 outlines the federal requirements that help to ensure that electronic records are trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper.
The first step in becoming compliant with the regulations is to determine whether or not you are required to be compliant. The following questions can help lead to the determination:
-
Is your clinical trial conducted under an approved IND? If yes, electronic system needs to meet part 11
-
Is your clinical trial conducted under an approved IDE? If yes, electronic system needs to meet part 11
-
Does your grant specify that your computer systems must comply with 21 CFR Part 11 or similar requirements? If yes, electronic system needs to meet part 11
If you answered yes to any of these questions, your electronic systems of record that are being used to meet predicate rules are required to comply with 21 CFR Part 11. Your sponsor may provide you with a system to use. The two options available at Pen Medicine are Penn CMTS and Veeva Vault EDC.