Tissue Bank
- The SCXC serves as a center for the collection and banking of live cells and plasma from peripheral blood, bone marrow, and pheresis products collected from hematological malignancy patients treated at the Hospital of the University of Pennsylvania (HUP). Samples are collected in accordance with IRB protocol 703185.
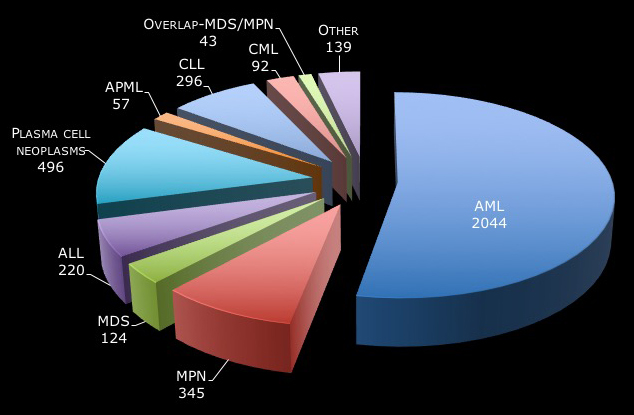
- The chart below shows the number of patients represented in our biospecimen bank for each type of hematological malignancy.
- For consultation or questions, please contact Marty Keoughor Martin Carroll.
- Each biospecimen is catalogued in a database and linked to annotations that include diagnosis, age, gender, ethnicity, cytology, cytogenetics, mutation analysis (up to 67 mutations for AML), and clinical immunophenotyping. For some leukemia samples, data related to their capacity to engraft in immune-deficient mice is available
- De-identified biospecimens and annotations are made available to researchers on a fee-for-service basis. Researchers do not have direct access to our database but availability of samples with very specific characteristics (multiple mutations) can be determined by contacting our tissue bank staff.
- In response to user demand for additional characterization of our biospecimens, we can develop and validate custom flow cytometry panels for applications such as target identification and quantification on large and diverse cohorts of patient samples
- Of note, sample requests are reviewed and approved by the Medical Director of the SCXC prior to the release of the samples to the investigators. Please also note that the samples are not consented for whole genome sequencing (WGS) or other research that would potentially yield to patient identification. Accordingly, requests for such experiments or analyses will be systematically denied.
- Collection, banking and distribution of bone marrow mononuclear cells from healthy donors
- Collection, banking and distribution of mononuclear cells from umbilical cord blood
- Purification, banking and distribution of CD34+ cells isolated from healthy donor bone marrow, cord blood and FL
- Of note, sample requests are reviewed and approved by the Medical Director of the SCXC prior to the release of the samples to the investigators. Please also note that the samples are not consented for whole genome sequencing (WGS) or other research that would potentially yield to patient identification. Accordingly, requests for such experiments or analyses will be systematically denied.
- Access to high-throughput immunomagnetic cell selection device (autoMACS Pro). For more information on autoMACS Pro, please visit the autoMacs page located within the Services tab on our website.
- Custom cell purification services for patient specimens

