Service Providers and Third Parties
Site: Third Party Selection and Management
A vendor or Service Provider under ICH E6R2 is a person or organization (commercial, academic, or other) providing a service (i.e., running a unique assay or imaging subjects for a trial) to either the sponsor or the investigator to fulfil one or more of their trial-related activities. Utilizing appropriately trained and qualified vendors ensures that investigators conducting their trials are in alignment with ICH E6 (R2) Good Clinical Practice Guidelines. While vendors may conduct essential trial functions, the ultimate responsibility for the quality and integrity of the study falls on the Investigator. To this end, Investigators have an interest in assisting the sponsor with vendor oversight.
Sponsor: Third Party Selection and Management
A vendor (or Service Provider under ICH E6R3) is a person or organization (commercial, academic, or other) providing a service to either the sponsor or the investigator to fulfil one or more of their trial-related activities. A sponsor may transfer any or all of their trial-related duties and functions to a vendor or other service provider, but the ultimate responsibility for the sponsor’s trial-related activities, including protection of participants’ rights, safety and well-being and reliability of the trial data resides with the sponsor. Prior to the transfer of any trial-related duty and function to a vendor or service provider, the Sponsor shall ensure that the transferee is qualified through documented training, experience or a combination thereof prior to transfer of responsibilities. Any trial related duty or function that is transferred to and assumed by a non-Penn entity must be specified in writing. The nature and scope of this writing will be determined upon submission to either the Research Information System (RIS) request or PennERA submission.
See drop downs below for additional information on selecting and managing third party vendors
When selecting a service provider, you should first determine the criteria for selecting that vendor. Begin by determining what requisite skills, facilities, attributes, and resources are required for conducting the contracted activities. Approved and preferred vendors, as determined by Penn Procurement Services, should be considered first for materials or services. If an approved or preferred vendor does not suit the needs of the activity/service/obligations or material, consult with Strategic Sourcing in Penn Procurement Services.
The Sponsor and/or PI should ensure that all trial processes delegated to a vendor are conducted in compliance with the Protocol and related documents. The range and extent of oversight measures should be fit for purpose and tailored to the complexity of and risks associated with the trial. Oversight should ensure quality. The Office of Clinical Research recommends that at a minimum, each vendor be qualified prior to utilization and annually, thereafter, to confirm that the vendor continues to maintain the ability and capacity to perform the delegated responsibility.
For additional information on Sponsor Vendors please review PSOM SOP 002 and the OCR Vendor Qualification Guide and utilize the OCR resources below for selection and documentation of a vendor qualifications. For additional information regarding qualifications and sponsor vendor oversight, please contact OCR Regulatory.
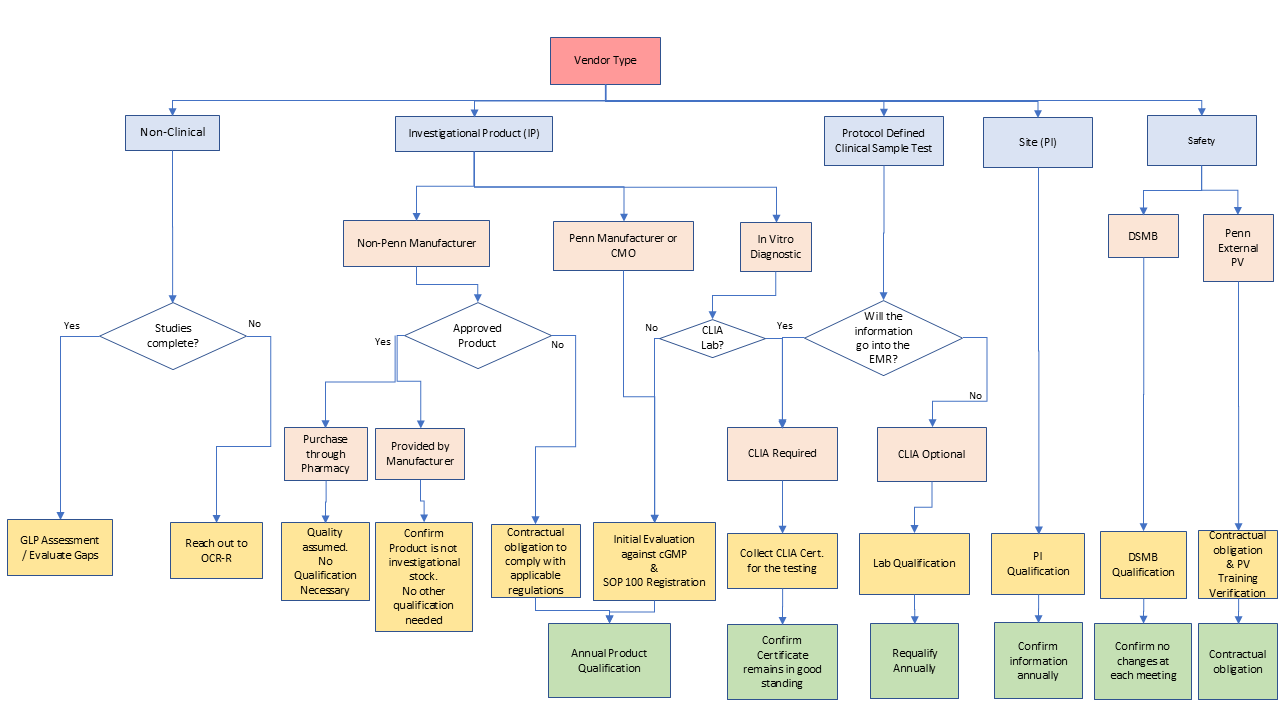
Below is a decision tree to help assist the sponsor in determining quality oversight of vendors prior to activation and during a clinical trial.
References
- Manufacturers or product release testing facilities see Investigational Product Management
- Central Laboratories or Clinical Sample Testing Facilities: Qualifying a Laboratory Performing Human Sample Testing and Penn Medicine Guidance on Sharing Data and Biological Samples with Third Parties
- For non-CLIA laboratories utilize, Non-CLIA GLP Vendor Qualification
- Investigators as a vendor - PI Qualification Form, Site Financial Disclosure Form & Site Qualification Report
- Data Safety Monitoring Board
- Clinical Research Organization (CRO) including monitoring, database, data management, and clinical operations services, contact OCR Operations.