- Home
- Shared Resources
- Rodent Cardiovascular Phenotyping Core
 The Rodent Cardiovascular Phenotyping Core provides echocardiographic services to assess cardiovascular function in mouse models and to provide validated surgical models of heart and vascular disease in mice to assess genetic or therapeutic interventions. We will work with investigators to design and implement the study along with any associated grant applications, animal protocol submissions and manuscript preparation. While the central focus of the facility is cardiovascular research, the techniques employed are often useful to investigators in other fields. Our Core staff will work with you to assess your needs and provide the necessary technical training and scientific assistance in animal protocol preparation. The Core runs on a fee-for-service model. Accordingly, investigators will be responsible for the costs incurred for their projects and prior animal protocol approval by Penn IACUC.
The Rodent Cardiovascular Phenotyping Core provides echocardiographic services to assess cardiovascular function in mouse models and to provide validated surgical models of heart and vascular disease in mice to assess genetic or therapeutic interventions. We will work with investigators to design and implement the study along with any associated grant applications, animal protocol submissions and manuscript preparation. While the central focus of the facility is cardiovascular research, the techniques employed are often useful to investigators in other fields. Our Core staff will work with you to assess your needs and provide the necessary technical training and scientific assistance in animal protocol preparation. The Core runs on a fee-for-service model. Accordingly, investigators will be responsible for the costs incurred for their projects and prior animal protocol approval by Penn IACUC.
Staff
Ling Lai, M.D., Ph.D.
Director and Cardiac Surgeon
Phone: 215-898-2656
Email: linglai@pennmedicine.upenn.edu
Surgical Suite: Smilow 11-222
Joanna Griffin, MS, RCS, RDCS
Rodent Echocardiographer
Phone: 215-573-3913
Email: Joanna.Griffin@Pennmedicine.upenn.edu
Echo Lab: Smilow 11-222
Ingrid Marti Pamies, PhD
Mouse Echocardiographer
Email: ingridm@pennmedicine.upenn.edu
Marielle Scherrer-Crosbie, MD, PhD
Faculty Core Advisor for Echocardiography
Email: Marielle.Scherrer-Crosbie@pennmedicine.upenn.edu
Office: South Pavilion 11-131
Daniel P. Kelly, MD
Faculty Core Advisor for Cardiac Surgery and Physiology
Email: dankelly@pennmedicine.upenn.edu
Office: Smilow TRC 11-122
Operations:
Teresa C. Leone
Director, Research Administration and Operations, CVI
Phone: 215-898-0768
Email: terleone@pennmedicine.upenn.edu
Office: Smilow 11-116
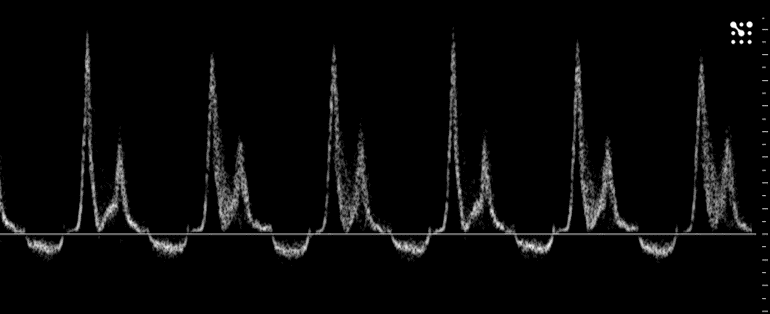
Cost is per mouse/per time point. Non-invasive single transthoracic cardiac ultrasound exam performed under Avertin (tribromoethanol)-induced anesthesia.
Users can consider A for screening mice for homogenous decreases in function /hypertrophy (e.g. sepsis, etc.). Consider D in mice with regional wall motion abnormalities. Note that M Mode parameters do not accurately represent LV structure and function.
- SCREENING ECHO
Acquisition and analysis of M-Mode images for LV function (fractional shortening), LV chamber dimensions, and LV wall thickness. Ideal for initial assessment of genetically-engineered mouse models. - SCREENING PLUS ECHO
Includes all screening echo parameters as well as acquisition and analysis of 2 dimensional images for LV end-diastolic and end-systolic volumes and ejection fraction. - ECHO for Advanced Diastolic Function Analysis*
Includes all screening plus echo parameters as well as acquisition and analysis for diastolic function: Doppler of Mitral valve inflow, Tissue Doppler imaging of septal and lateral walls, Doppler of pulmonary vein flow, and LV cardiac strain (LV volumes, EF, LV Mass). - TAC ECHO
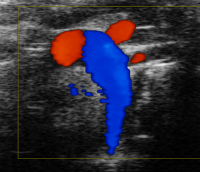
Screening Plus echo (M-Mode, volumes, EF) in addition to aortic trans-banding gradient assessment. - MI ECHO
Acquisition of 2D long axis images for cardiac strain (LV volumes, EF, LV Mass) and 2D short axis serial imaging slices for regional wall motion analysis. - TAC+ MI (Heart Failure) ECHO
MI echo plus aortic trans-banding gradient assessment. - Right heart assessment (PAB ECHO)
Acquisition of 2D and Doppler images for assessment of RV area, RV free wall thickness, RV tissue Doppler imaging, TAPSE, tricuspid regurgitation, and pulmonary artery band gradient.
*Consultation with Core required prior to scheduling.
- TRANSVERSE AORTIC CONSTRICTION (TAC)- Compensated LV Hypertrophy Model
Operative placement of constriction on transverse aorta that results in left ventricular hypertrophy with normal systolic function in timeframe of 1-4 weeks. Timing of endpoint determined by scientific question. Recommend M-mode echo with gradient at endpoint. - MYOCARDIAL INFARCTION (MI)
Operative placement of a permanent occlusion around the left anterior descending artery of the mouse heart. Includes recording mouse weight. Size of MI can be customized but is typically sufficient size to impact LV function. Recommended Complete 2D echo and collection of endpoints at 1-2 weeks post-surgery. - OPEN CHEST ISCHEMIA REPERFUSION INJURY (OCIR)
Operative placement of an occluder around the left anterior descending artery for 30-45mins of occlusion followed by reperfusion for typically 24hrs. Vital dye staining will also be performed which involves euthanasia and placement of infusion line in aorta, and ligation of innominate artery, carotid artery and left subclavian artery. Heart will then be perfused with tetrazolium chloride and Evans Blue, with analysis to determine infarcted area and “area at risk”. -
CLOSED CHEST MYOCARDIAL ISCHEMIA REPERFUSION (CCIR)
1-2 weeks prior to the ischemic event, a suture is placed around the left anterior descending artery and threaded through a short piece of plastic tubing to create a loose occluder. On the day of the ischemia, the chest is re-opened, the suture is pulled tight for 60-90 mins, followed by reperfusion. The ischemic event is confirmed by ECG and echocardiography to determine area at risk. This CCIR model enables the investigator to study the effects of ischemia and reperfusion in both the acute and chronic setting, allowing flexibility in choosing the duration and the frequency of ischemia, and avoids background noise related to thoracotomy induced inflammation.
- COMBINED TRANSVERSE AORTIC CONSTRICTION (TAC)/MYOCARDIAL INFARCTION (MI) – Heart Failure (HF) Model
Operative placement of constriction on the transverse aorta combined with a small myocardial infarction caused by permanent ligation of the distal third of the left anterior descending artery. Heart Failure (global LV dilatation and remodeling) will typically progress over a 4 week period. Recommend Complete 2D echo at endpoint. - PULMONARY ARTERY BANDING (PAB)
Operative placement of constriction on the pulmonary artery. RV dilation, dysfunction, and tricuspid regurgitation will typically progress over a 7-10 day period. We recommend a PAB echo be performed at day 10 post surgery. - PRE-WEANING AORTIC BANDING (PWAB)
Operative placement of constriction on the ascending aorta at 3 weeks of age. Recommend imaging at 8-10 weeks post-surgery (chronic model). PWAB results in a profound left ventricular hypertrophy and late-stage diastolic dysfunction with normal systolic function.
OTHER SERVICES
- INJECTIONS OF PHARMACOLOGICAL AGENTS
- JUGULAR CATHETERIZATION (for drug infusion)
- ALZET PUMP IMPLANTATION
- TELEMETRY IMPLANTATION
- ORGAN HARVEST
- HEMODYNAMICS I
Millar catheter placement, in carotid/aorta and recording of arterial or aortic pressures only. - HEMODYNAMICS II
Millar catheter placement in left or right ventricle, and derivation of ventricular peak pressures, ventricular end-diastolic pressure, +/- dP/dt, and tau. - HEMODYNAMICS III
Millar catheter placement into left ventricle and recording of LV measurements as in “Hemodynamics II” during pharmacological intervention (Dobutamine Challenge). - HEMODYNAMICS IV
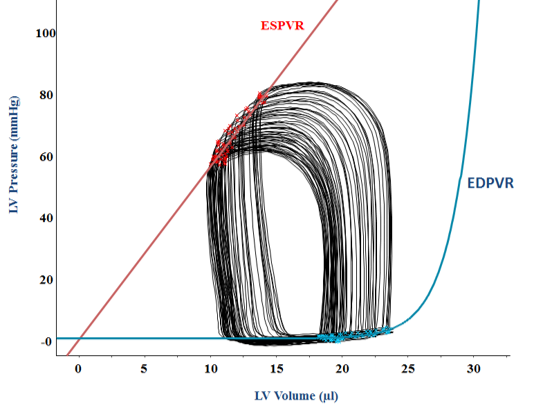
Millar catheter placement into left ventricle, Pressure-Volume loop recording including all measurements of “Hemodynamics II”.
-
Request Surgical, Echo, and Hemodynamics Services
-
Prices for Surgical, Echo, and Hemodynamics Services
-
CVI Rodent Cardiovascular Phenotyping Core Mouse and Data Transfer Logsheet
-
Slides from Rodent Cardiovascular Phenotyping Core Presentation
Other services are available upon request. Please email terleone@pennmedicine.upenn.edu.
Please remember to acknowledge the RCPC in your publications as follows:
- For cardiac/hemodynamics services: The cardiac surgeries (or hemodynamic studies) were performed by the Rodent Cardiovascular Phenotyping Core (RRID: SCR_022419) at the University of Pennsylvania supported by the Penn Cardiovascular Institute.
- For echo services: The echocardiography were performed by the Rodent Cardiovascular Phenotyping Core (RRID: SCR_022419) at the University of Pennsylvania supported by the Penn Cardiovascular Institute and NIH S10OD016393.
Selected Publications
1. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature., 2019 Sep 573(7774):430-433. doi: 10.1038/s41586-019-1546-z. Epub. PMID: 31511695
2. Salva R Yurista , Timothy R Matsuura, Herman H W Silljé, Kirsten T Nijholt , Kendra S McDaid , Swapnil V Shewale, Teresa C Leone, John C Newman, Eric Verdin , Dirk J van Veldhuisen , Rudolf A de Boer , Daniel P Kelly , B Daan Westenbrink. Ketone Ester Treatment Improves Cardiac Function and Reduces Pathologic Remodeling in Preclinical Models of Heart Failure. Circ Heart Fail., 2021 Jan 14(1):e007684. PMID: 33356362 PMCID: PMC7819534
3. Tsunehisa Yamamoto, Santosh K Maurya, Elizabeth Pruzinsky, Kirill Batmanov, Yang Xiao, Sarah M Sulon, Tomoya Sakamoto, Yang Wang, Ling Lai, Kendra S McDaid, Swapnil V Shewal, Teresa C Leone, Timothy R Koves, Deborah M Muoio, Pieterjan Dierickx, Mitchell A Lazar, E Douglas Lewandowski, Daniel P Kelly. RIP140 deficiency enhances cardiac fuel metabolism and protects mice from heart failure. J Clin Inves., t 2023 May 1;133(9):e162309. PMID: 36927960 PMCID: PMC10145947
4. Danielle Murashige, Jae Woo Jung, Michael D Neinast, Michael G Levin, Qingwei Chu, Jonathan P Lambert, Joanne F Garbincius, Boa Kim, Atsushi Hoshino, Ingrid Marti-Pamies, Kendra S McDaid, Swapnil V Shewale, Emily Flam, Steven Yang, Emilia Roberts, Li Li, Michael P Morley, Kenneth C Bedi Jr, Matthew C Hyman, David S Frankel, Kenneth B Margulies, Richard K Assoian, John W Elrod, Cholsoon Jang, Joshua D Rabinowitz, Zoltan Arany. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab., 2022 Nov 1;34(11):1749-1764.e7. PMID: 36223763 PMCID: PMC9633425
5. Lun Li , Aileen A Ren, Siqi Gao, Yourong S Su, Jisheng Yang, Jenna Bockman, Patricia Mericko-Ishizuka, Joanna Griffin, Robert Shenkar, Roberto Alcazar, Thomas Moore, Rhonda Lightle, Dorothy DeBiasse, Issam A Awad, Douglas A Marchuk, Mark L Kahn, Jan-Karl Burkhardt. mTORC1 Inhibitor Rapamycin Inhibits Growth of Cerebral Cavernous Malformation in Adult Mice. Stroke. 2023 Nov;54(11):2906-2917. PMID: 37746705 PMCID: PMC10599232
6. Yamamoto T, Maurya SK, Pruzinsky E, Batmanov K, Xiao Y, Sulon SM, Sakamoto T, Wang Y, Lai L, McDaid KS, Shewale SV, Leone TC, Koves TR, Muoio DM, Dierickx P, Lazar MA, Lewandowski ED, Kelly DP: RIP140 deficiency enhances cardiac fuel metabolism and protects mice from heart failure. J Clin Invest., 2023 May 1;133(9):e162309. PMID: 36927960; PMCID PMC10145947
7. Li L, Ren AA, Gao S, Su YS, Yang J, Bockman J, Mericko-Ishizuka P, Griffin J, Shenkar R, Alcazar R, Moore T, Lightle R, DeBiasse D, Awad IA, Marchuk DA, Kahn ML, Burkhardt JK. mTORC1 Inhibitor Rapamycin Inhibits Growth of Cerebral Cavernous Malformation in Adult Mice. Stroke, 2023 Nov;54(11):2906-2917. doi: 10.1161/STROKEAHA.123.044108. Epub 2023 Sep 25. PMID: 37746705
8. Kim B, Zhao W, Tang SY, Levin MG, Ibrahim A, Yang Y, Roberts E, Lai L, Li J, Assoian RK, FitzGerald GA, Arany Z. Endothelial lipid droplets suppress eNOS to link high fat consumption to blood pressure elevation. J Clin Invest., 2023 Dec 15;133(24):e173160. doi: 10.1172/JCI173160. PMID: 37824206 Free PMC article.
9. Kim K, Kim MM, Skoufos G, Diffenderfer ES, Motlagh SAO, Kokkorakis M, Koliaki I, Morcos G, Shoniyozov K, Griffin J, Hatzigeorgiou AG, Metz JM, Lin A, Feigenberg SJ, Cengel KA, Ky B, Koumenis C, Verginadis II. FLASH Proton Radiation Therapy Mitigates Inflammatory and Fibrotic Pathways and Preserves Cardiac Function in a Preclinical Mouse Model of Radiation-Induced Heart Disease. Int J Radiat Oncol Biol Phys., 2024 Jul 15;119(4):1234-1247. doi: 10.1016/j.ijrobp.2024.01.224. Epub 2024 Feb 15. PMID: 38364948
10. Berger J, Matsuura TR, Bowman CE, Taing R, Patel J, Lai L, Leone TC, Reagan JD, Haldar SM, Arany Z, Kelly DP: Sodium-glucose co-transporter 2 Inhibitors Act Independently of SGLT2 to Confer Benefit for heart failure with reduced ejection fraction in mice. bioRxiv, 2024.04.29.591665 PMID: 38746425
11. Berger JH, Shi Y, Matsuura TR, Batmanov K, Chen X, Tam K, Marshall M, Kue R, Patel J, Taing R, Callaway R, Griffin J, Kovacs A, Shanthappa DH, Miller R, Zhang BB, Roth Flach RJ, Kelly DP. Two-hit mouse model of heart failure with preserved ejection fraction combining diet-induced obesity and renin-mediated hypertension. bioRxiv, 2024 Jun 9:2024.06.06.597821 PMID: 38895483
12. Choe D, Burke M, Brandimarto JA, Marti-Pamies I, Yob J, Yang Y, Morley MP, Drivas TG, Day S, Damrauer S, Wang X, Cappola TP. Sex-Specific Effect of MTSS1 Downregulation on Dilated Cardiomyopathy. medRxiv [Preprint]. 2024 Mar 1:2024.02.28.24303451. doi: 10.1101/2024.02.28.24303451. PMID: 38464240
13. Eaton DM, Lee BW, Caporizzo M, Iyengar A, Chen CY, Uchida K, Marcellin G, Lannay Y, Vite A, Bedi Jr KC, Brady CF, Smolyak JN, Meldrum D, Dominic J, Weingarten N, Patel M, Belec A, Hached K, Atluri P, Van Der Lann S, Prosser B, Margulies KB. Vasohibin inhibition improves myocardial relaxation in a rat model of heart failure with preserved ejection fraction. Science Translational Medicine, 2024, July 17;16(756):eadm8842. PMID: 39018366
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser. | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team. | Sitemap


