Ongoing Research Projects
Acute and chronic pathologies to soft tissues of the joint represent a tremendous socioeconomic problem. As traditional treatments can often lead to inconsistent and undesirable outcomes, there is an unmet clinical need to develop therapies to repair injuries to these tissues and attenuate the progression of chronic pathologies. However, it has been difficult to devise therapies because of a gap in knowledge regarding the processes that regulate the formation and repair of these tissues. Our research program aims to better define the mechanisms that regulate normal growth and development compared to impaired healing and/or chronic pathogenesis in the adult to devise improved strategies to treat injury and disease of these tissues.
K99/R00 AR067283
PI: Nathaniel Dyment
Title: Defining the Tendon Lineage to Improve Tissue Engineering Strategies
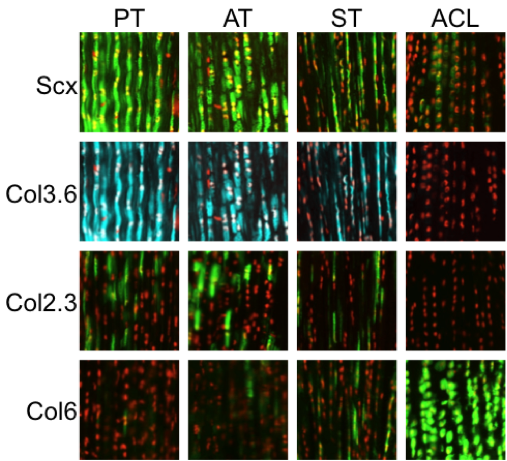
Normal tendon consists of a hierarchical structure consisting of collagen fibrils assembled into collagen fibers and fascicles surrounded by interstitial endo-, epi-, and paratenon structures. Cells within the collagen fascicles have traditionally been defined as internal tendon fibroblasts or tenocytes. It was initially believed that these cells were a homogeneous population but current research suggests that these cells may be more heterogeneous than previously thought. However, the biological tools to map out these differences have eluded the field thus far. We have developed a repertoire of lineage tracing and GFP reporter mice that identify resident tendon populations and showcase the heterogeneity of cells in the tendon midsubstance and enthesis. We aim to characterize the expression profile of resident tendon progenitors as they differentiate during normal processes of tendon growth and natural healing following injury. The central hypothesis is that the molecular mechanisms that drive tendon progenitor differentiation during growth are crucial to improving tendon repair in the adult. Therefore, adult progenitors that mimic the “growth” differentiation profile during “repair” will lead to improved mechanical outcome. We have two inducible Cre models that label potential progenitor cell populations within the tendon body during growth. In addition, these models label cell populations within the surrounding epi/paratenon that contribute to tendon healing and repair. These inducible Cre models will be used in combination with other GFP reporter mouse models that label subsets of cells within the tendon midsubstance. We will define the expression profiles that delineate these subpopulations to map the heterogeneity of the internal tendon fibroblast population. Using the expression profiles and markers defined in earlier studies, we will isolate resident progenitors from mouse and human tendon tissue. The reparative potential of these cell populations will be compared in tendon defects of immunocompromised mice. If successful, this research will:
- help characterize the resident tendon progenitors that respond to injury,
- help isolate progenitor cell sources conducive for tendon repair, and
- provide biological success criteria for tenogenic differentiation.
|
|
For More Information See:
- Dyment NA, Kazemi N, Aschbacher-Smith LE, Barthelery NJ, Kenter K, Gooch C, Shearn JT, Wylie C, Butler DL. The relationships among collagen gene expression, histology, and biomechanics following full-length injury in the patellar tendon. J Orthop Res. 2012. 30(1):28-36. PMCID: PMC3181390.
- Dyment NA, Liu CF, Kazemi N, Aschbacher-Smith LE, Kenter K, Breidenbach AP, Shearn JT, Wylie C, Rowe DW, Butler DL. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLOS ONE. 2013. 8(3):e59944. PMCID: PMC3608582.
- Breidenbach AP, Gilday SD, Lalley AL, Dyment NA, Gooch C, Shearn JT, Butler DL. Functional tissue engineering of tendon: Establishing biological success criteria for improving tendon repair. J Biomech. 2014. 47(9):1941-1948. PMCID: PMC3995907.
- Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLOS ONE. 2014. 9(4):e96113. PMCID: PMC3997569.
- Dyment NA, Galloway JL, Regenerative biology of tendon: mechanisms for renewal and repair. Curr Mol Bio Rep. 2015. 1(3):124-131. PMCID: PMC4570727
- Wall ME, Dyment NA, Bodle J, Volmer J, Loboa E, Cederlund A, Fox AM, Banes AJ. Cell Signaling in Tenocytes: Response to Load and Ligands in Health and Disease. Adv Exp Med Biol. 2016;920:79-95. DOI: 10.1007/978-3-319-33943-6_7. PMID: 27535250.
- Yoshida R, Alaee F, Dyrna F, Kronenberg MS, Maye P, Kalajzic I, Rowe DW, Mazzocca AD, Dyment NA, Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res. 2016. 57(6):507-515. PMID: 27184388.
Development, maturation, and perturbation of the zonal tendon-to-bone enthesis
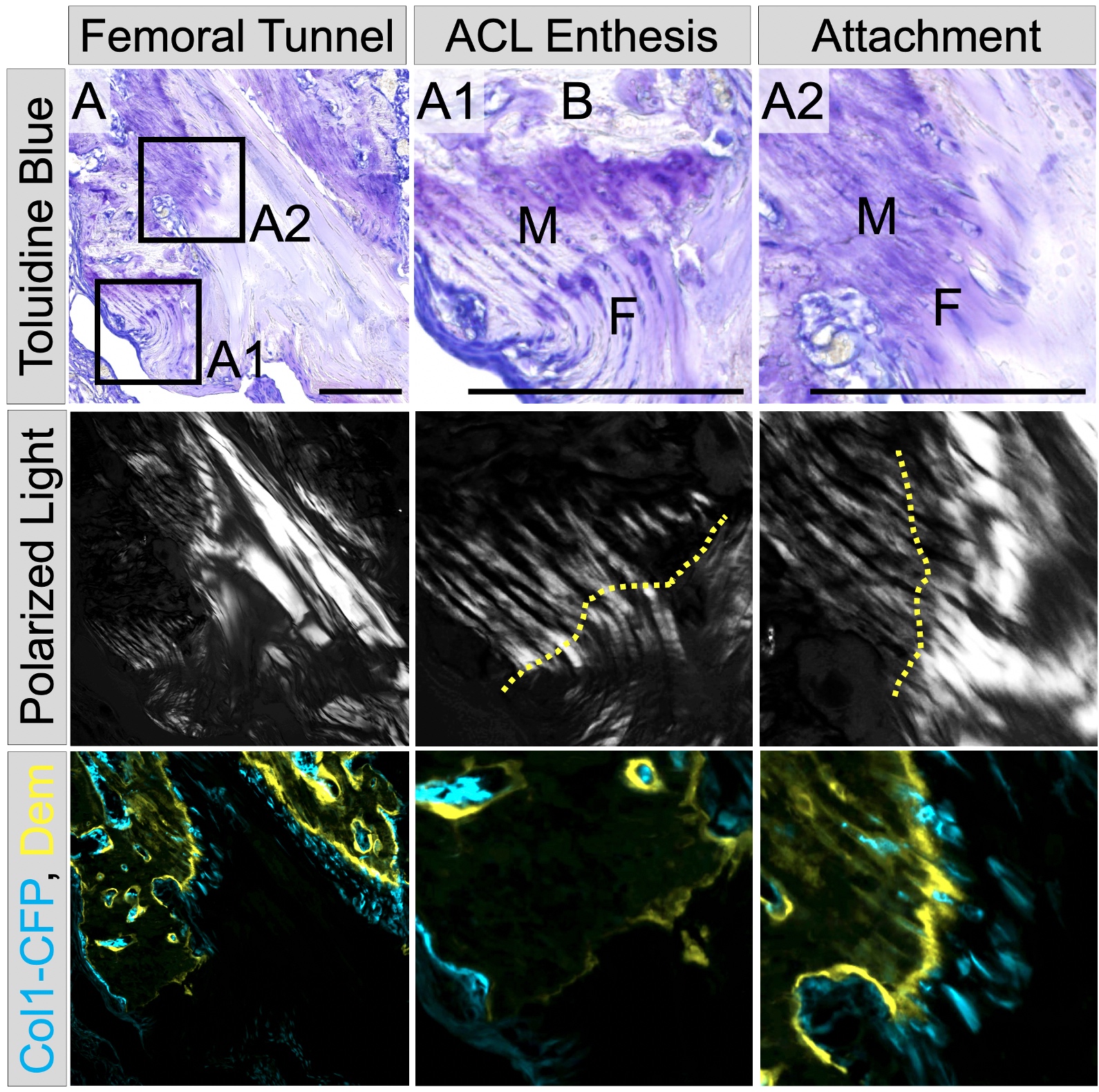
The interfaces that connect tendons and ligaments to bone, known as entheses, are crucial for efficient load transfer and subsequent ambulation of the skeleton. Unlike the myotendinous junction, the enthesis is the prevalent location for a variety of tendon/ligament injuries and chronic pathologies. The fibrocartilaginous enthesis is a zonal structure consisting of the tendon/ligament midsubstance, unmineralized fibrocartilage, mineralized fibrocartilage, and underlying bone. Our lab focuses on the coordinated events that lead to the formation of these zones, particularly the mineralized fibrocartilage, and how this process is impaired during repair or altered in chronic pathologies.
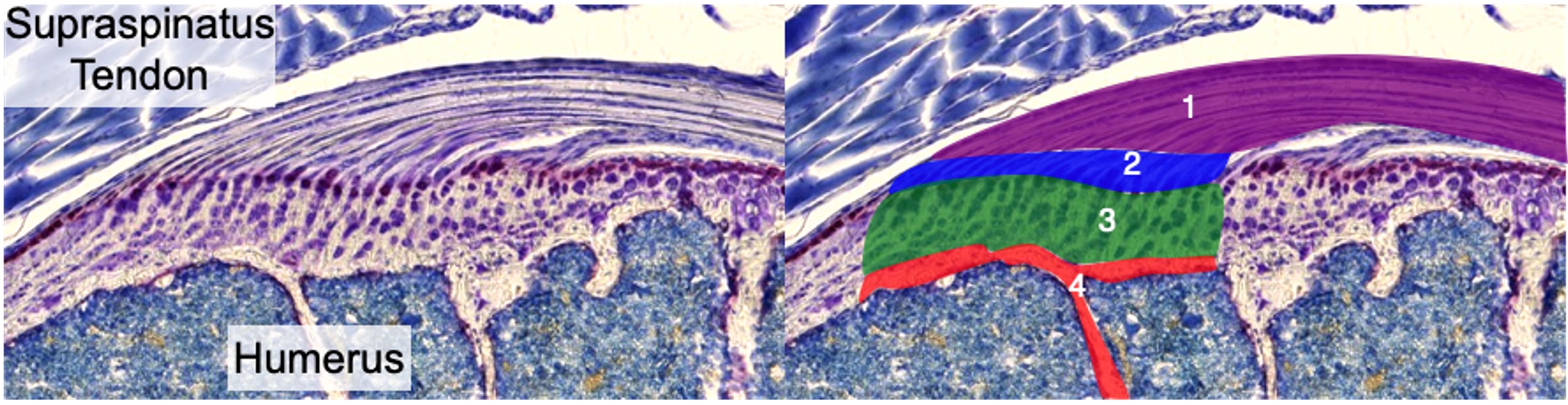
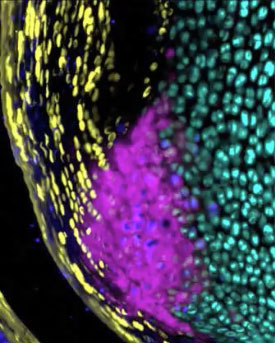
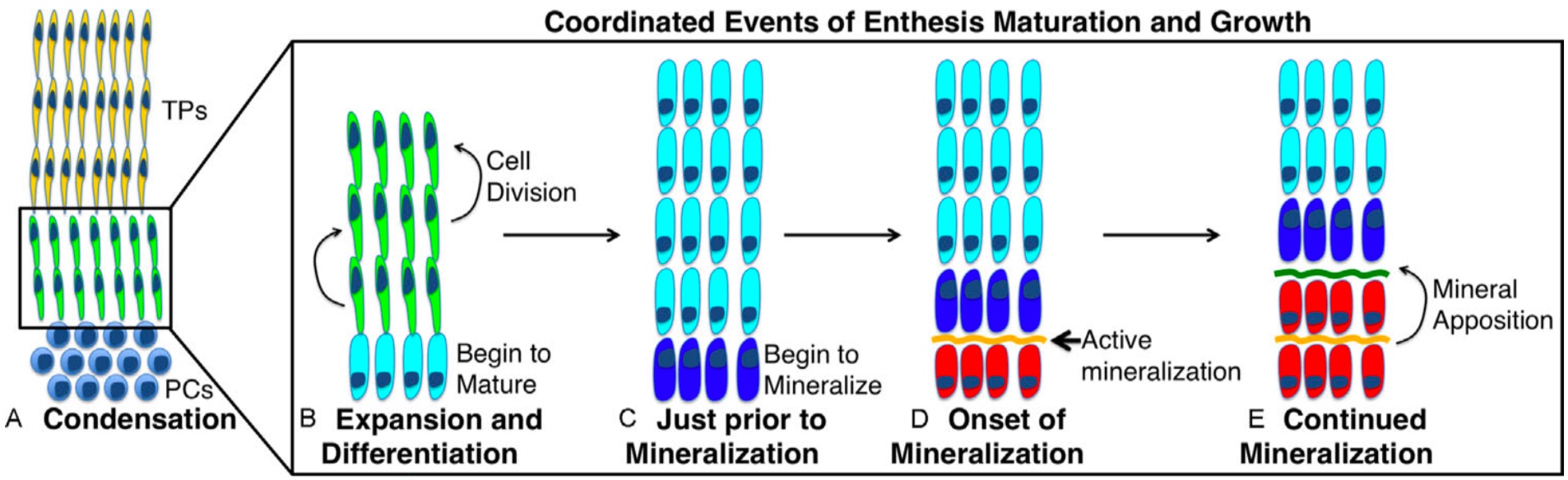
For normal growth and development, our lab is interested in understanding the contribution of cell proliferation and matrix production to the growth of the enthesis tissue. In addition, we focus on defining markers that define the maturation of these cells from progenitors to unmineralized fibrochondrocytes and then finally mineralized fibrochondrocytes. Finally, we investigate the signaling pathways that regulate these processes. This strategy of defining the cellular/matrix dynamics of enthesis formation and the mechanisms that regulate it will guide future repair therapies in our laboratory.
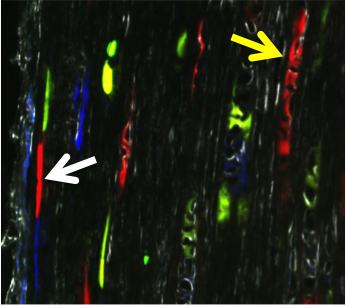
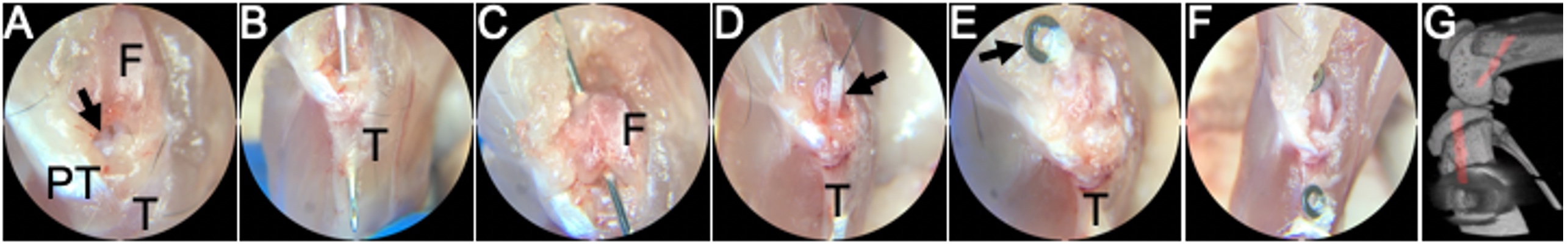
Anterior cruciate ligament (ACL) reconstruction is a common procedure following an ACL tear. While this procedure is generally successful, two clinical needs that exist are 1) improving ligamentization or revitalization of the graft tissue and 2) improving tendon-to-bone integration in the tunnels. Our lab utilizes a novel murine ACL reconstruction model to better understand the processes that contribute to these clinical needs. The aims are to identify the markers that define the progenitor cells populations that revitalize the graft and to improve their ability to infiltrate into the graft and differentiate properly. In addition, we will compare the biological processes that lead to tendon-to-bone attachments in this repair model and compare them to the process that we defined during enthesis formation in growth and development.
For More Information See:
- Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLOS ONE. 2014. 9(4):e96113. PMCID: PMC3997569.
- Dyment NA, Hagiwara Y, Jiang X, Huang J, Adams DJ, Rowe DW. Response of knee fibrocartilage to joint destabilization. Osteoarthritis Cartilage. 2015. 23(6):996-1006. PMID: 25680653.
- Breidenbach AP, Aschbacher-Smith L, Lu Y, Dyment NA, Liu CF, Wylie C, Rao M, Shearn JT, Rowe DW, Kadler KE, Jiang R, Butler DL. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res. 2015. 33(8):1142-1151. PMID: 25807894.
- Dyment NA, Breidenbach A, Schwartz A, Russell R, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, Butler DL, Rowe DW. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. 2015. 405(1):96-107. PMCID: PMC4529782 (Cover article)
- Yoshida R, Alaee F, Dyrna F, Kronenberg MS, Maye P, Kalajzic I, Rowe DW, Mazzocca AD, Dyment NA, Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res. 2016. 57(6):507-515. PMID: 27184388.
- Dyment NA, Jiang X, Chen L, Hong SH, Adams DJ, Ackert-Bicknell C, Shin DG, Rowe DW. High-throughput, multi-image cryohistology of mineralized tissues. J Vis Exp. 2016. (115):e54468, DOI: 10.3791/54468.
NIH R01 AR076381
PI: Nathaniel Dyment
Title: Functional role and therapeutic potential of hedgehog signaling in tendon-to-bone repair
During growth and development, a series of coordinated events lead to the formation of a mature zonal enthesis. This process begins when enthesis progenitor cells undergo clonal expansion and differentiation into unmineralized fibrochondrocytes. A subset of these fibrochondrocytes then begin to mineralize and form mineralized fibrocartilage, a process that our lab and others have shown is driven by hedgehog pathway (Hh) signaling. Together, these coordinated events lead to the formation of a zonal enthesis that effectively mitigates stress concentrations at the interface between soft tendon and hard bone via the established gradient in mineralization.
Despite its critical role during enthesis development, it is not known whether the Hh pathway plays a similar role in adult tendon-to-bone repair, representing a significant gap in knowledge. Unfortunately, studying these mechanisms during repair in the adult has been challenging, primarily because traditional tendon-to-bone reattachment surgeries do not produce zonal fibrocartilage. However, ligament reconstructions performed with a tendon graft passing through bone tunnels can result in zonal insertions. Bone marrow progenitor cells (BMPCs) express a-smooth muscle actin (aSMA) during expansion in response to the drilling of bone tunnels during surgery. We have previously demonstrated that these expanding aSMA-lineage cells are the primary contributors to tunnel integration; therefore, we can use genetic mouse models to target these cells and elucidate the role of Hh signaling during tendon-to-bone repair.
Our objective is to genetically and pharmacologically modulate the Hh pathway to define its functional role in the early expansion and later differentiation of cells that give rise to zonal tendon-to-bone attachments. Our central hypothesis is that the Hh pathway is a critical positive regulator of zonal enthesis formation in the adult and therefore stimulation of the pathway at both early and late time points will improve tendon-to-bone repair. Therefore, we will use genetic gain- and loss-of-function mouse models to target specific genes within the pathway as well as increase or decrease Hh signaling pharmacologically with small molecule Hh agonists or antagonists, respectively, during different stages of the repair process.
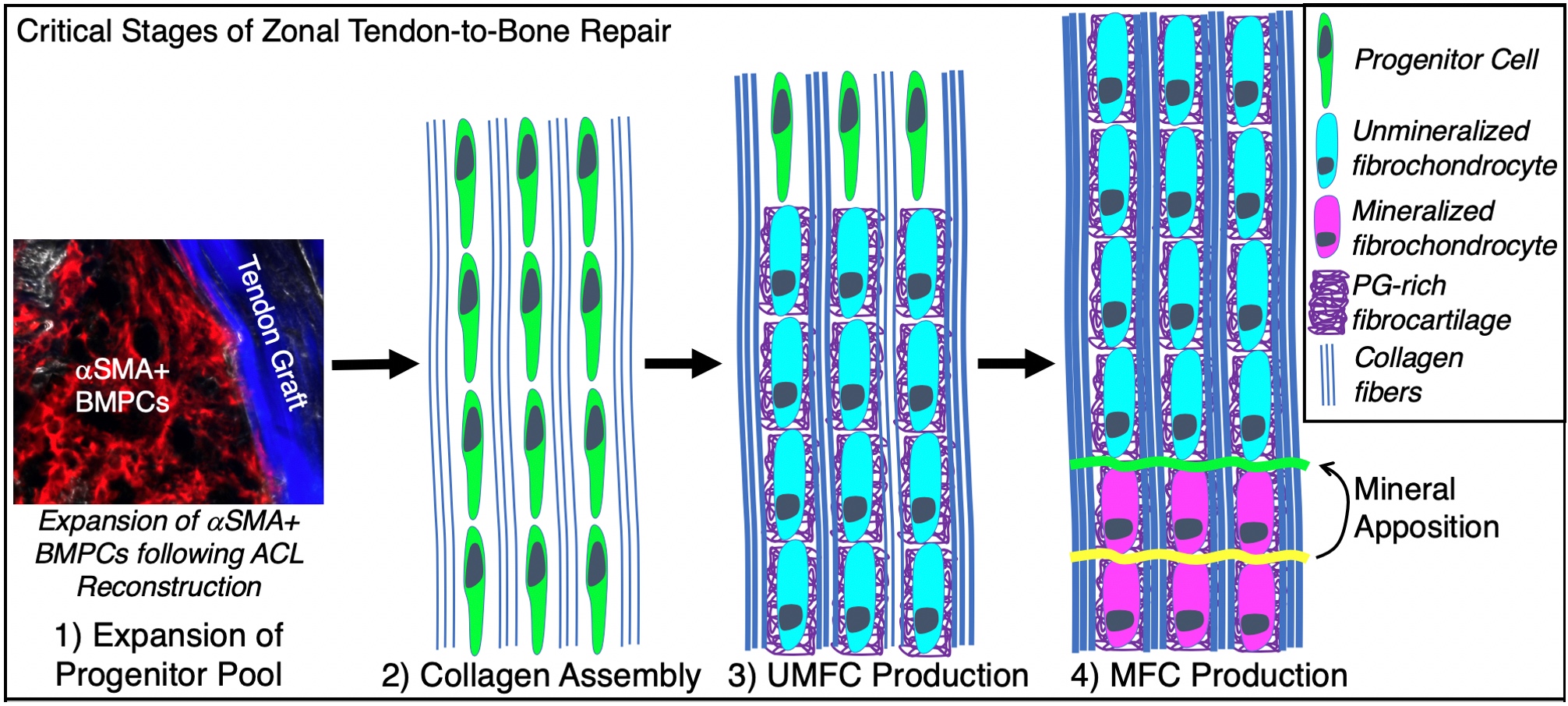
Micro computed tomography, multiplexed mineralized cryohistology, and laser-capture qPCR will be used to assess the effect these pathway modulations have on the four stages of the repair response. To assess the ultimate function of the repair, we will conduct anterior-posterior drawer tests and tunnel pullout tests. If successful, this research will define the role of Hh signaling during both the early and late stages of zonal tendon-to-bone attachment formation and will ultimately inform future therapeutic treatments to improve tendon-to-bone repair.
For more information see:
- Kamalitdinov TB, Fujino K, Shetye SS, et al. Amplifying Bone Marrow Progenitors Expressing α-Smooth Muscle Actin Produce Zonal Insertion Sites During Tendon-to-Bone Repair. J Orthop Res. 2020;38(1):105-116. doi:10.1002/jor.24395
- Hagiwara Y, Dyrna F, Kuntz AF, Adams DJ, Dyment NA. Cells from a GDF5 origin produce zonal tendon-to-bone attachments following anterior cruciate ligament reconstruction. Ann N Y Acad Sci. 2020;1460(1):57-67. doi:10.1111/nyas.14250
- Breidenbach AP, Aschbacher-Smith L, Lu Y, Dyment NA, Liu CF, Wylie C, Rao M, Shearn JT, Rowe DW, Kadler KE, Jiang R, Butler DL. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res. 2015. 33(8):1142-1151. PMID: 25807894.
- Liu CF, Breidenbach A, Aschbacher-Smith L, Butler D, Wylie C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS One. 2013;8(6):e65411. Published 2013 Jun 10. doi:10.1371/journal.pone.0065411
- Dyment NA, Breidenbach A, Schwartz A, Russell R, Aschbacher-Smith L, Liu H, Hagiwara Y, Jiang R, Thomopoulos S, Butler DL, Rowe DW. GDF5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. 2015. 405(1):96-107. PMCID: PMC4529782 (Cover article)
