Basic Biology of Kidney Disease
At the Penn/CHOP Kidney Innovation Center, our mission begins with a deep dive into the basic biology of kidney disease. This foundational research is the cornerstone of everything we do, guiding our understanding of disease mechanisms at the molecular level. By unraveling the complex biological processes that lead to kidney disease, our team aims to identify novel biomarkers and therapeutic targets. Our multidisciplinary approach, combining cutting-edge techniques and innovative methodologies, allows us to explore the kidney's intricate functions and responses to various stressors. This knowledge is crucial for developing preventive strategies and more effective treatments, bringing us one step closer to curing kidney diseases.
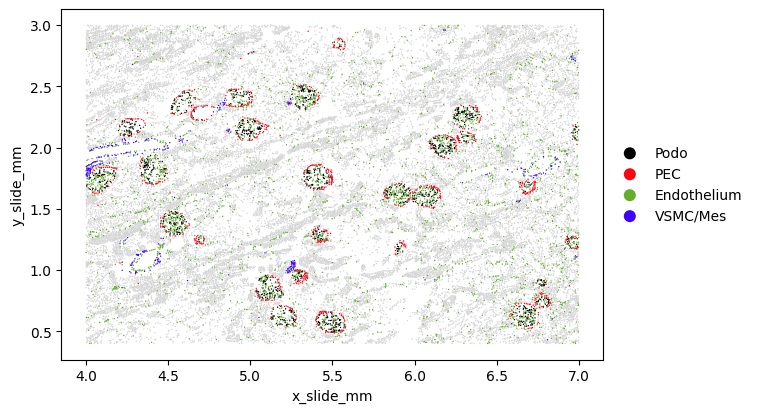
Overall Summary
The Basic Biology of Kidney Disease groups synthesizes the efforts of several groundbreaking studies:
Understanding Kidney Development, Regeneration, and Tissue Engineering
Our research at the Penn/CHOP Kidney Innovation Center extends into the fascinating realms of kidney development, regeneration, and tissue engineering. We are exploring the kidney's ability to repair and regenerate itself, critical to revolutionary treatments. Our team employs state-of-the-art bioengineering techniques alongside developmental biology insights to design new therapeutic approaches. By understanding how kidneys develop and heal, we aim to create innovative solutions for patients suffering from kidney diseases, potentially leading to the development of artificial kidneys and regenerative therapies that can restore kidney function. This study is led by:


Kidney Metabolism in Health and Disease
The research team led by Liming Pei, Joseph A. Baur, Daniel P Kelly, M. Celeste Simon, Zolt Arany, and Katalin Susztak is at the forefront of investigating kidney metabolism in health and disease states. Our work delves into how metabolic processes within the kidney adapt to various challenges and how disruptions in these processes can lead to disease. By understanding the metabolic pathways altered in kidney diseases, we aim to uncover new therapeutic targets and strategies to maintain kidney health. Our interdisciplinary approach combines genetics, molecular biology, and metabolic analysis to provide comprehensive insights into kidney metabolism, offering new hope for patients worldwide.






Understanding Kidney Disease through the Lens of Genetics, Genomics, and Multiomics
Our team, including Lisa Guy Woodford, Daniel Rader, Marylyn Ritchie, Katalin Susztak, and Parker Wilson, is pioneering research in understanding kidney disease through genetics, genomics, and multiomics approaches at the Penn/CHOP Kidney Innovation Center. This research aims to uncover the genetic and molecular underpinnings of kidney diseases by analyzing large datasets from diverse patient populations. Our work leverages cutting-edge technologies in sequencing and bioinformatics to identify genetic variants and molecular pathways that contribute to kidney disease. Through this comprehensive analysis, we are developing personalized medicine approaches that target specific genetic and molecular profiles, paving the way for more effective and tailored treatments.



PhD


Immune-mediated Kidney Disease
Our team brings together researchers with expertise in a broad range of autoimmune disease areas and experience that spans from the laboratory to clinical trials to patient treatment and care. By gathering these diverse talents around Penn’s growing Penn Colton Center for Autoimmunity, we are opening the door to a new frontier in autoimmune health. Our research aims to become the transformative epicenter of autoimmune research: the best scientific minds pursuing the most visionary projects with the most powerful resources at their fingertips. We envision that our studies will increase awareness and drive major and sustained progress in autoimmunity.





MD
From Kidney Disease to Kidney Cancer
Our research uniquely bridges the gap between kidney disease and kidney cancer. This innovative study area seeks to understand the progression from chronic kidney disease to kidney cancer, uncovering the biological links and molecular pathways that facilitate this transition. By identifying the risk factors and mechanisms contributing to kidney cancer development in patients with kidney disease, we aim to develop preventive strategies and targeted therapies. Our interdisciplinary research combines oncology, nephrology, urology, pathology and molecular biology, offering new insights and hope for patients facing the dual challenges of kidney disease and cancer.

Ph.D.







Machine Learning and Multi-omics Integration to Understand Kidney Disease
The integration of machine learning and multi-omics approaches offers a powerful framework for advancing our understanding of kidney disease. Multi-omics technologies, which include genomics, transcriptomics, proteomics, and metabolomics, enable comprehensive insights into the complex molecular mechanisms underlying kidney function and pathology. By integrating diverse omics layers, our studies aim to uncover previously unrecognized biological pathways, identify molecular signatures associated with disease progression, and predict patient outcomes more accurately. Our research holds immense potential for improving diagnosis, guiding personalized treatment, and ultimately enhancing our ability to prevent and manage kidney disease.

PhD

PhD

PhD


PhD

Machine Learning and Multi-omics Integration to Understand Kidney Disease
The integration of machine learning and multi-omics approaches offers a powerful framework for advancing our understanding of kidney disease. Multi-omics technologies, which include genomics, transcriptomics, proteomics, and metabolomics, enable comprehensive insights into the complex molecular mechanisms underlying kidney function and pathology. By integrating diverse omics layers, our studies aim to uncover previously unrecognized biological pathways, identify molecular signatures associated with disease progression, and predict patient outcomes more accurately. Our research holds immense potential for improving diagnosis, guiding personalized treatment, and ultimately enhancing our ability to prevent and manage kidney disease.

PhD

PhD

PhD


PhD



