Decentralized Trials
The format of decentralized trials was developed originally developed to serve resource constrained researchers across a growing network of sites and really expanded during the pandemic when traditional in-person visits and activities, simply were not possible.
In a decentralized clinical trial (DCT), some or all of a clinical trial's activities occur at locations other than a traditional clinical trial site. These alternate locations can include the participant's home, a local health care facility that is nearby, rather than a central site, or a nearby laboratory. They may also include an increased use of technology to support the research from recruitment, patient communication, all the way to ongoing data collection.
For patients, this provides levels of increased flexibility and allows for enrollment of patients who may live further away and/ or have travel or cost related issues associate with coming on site.
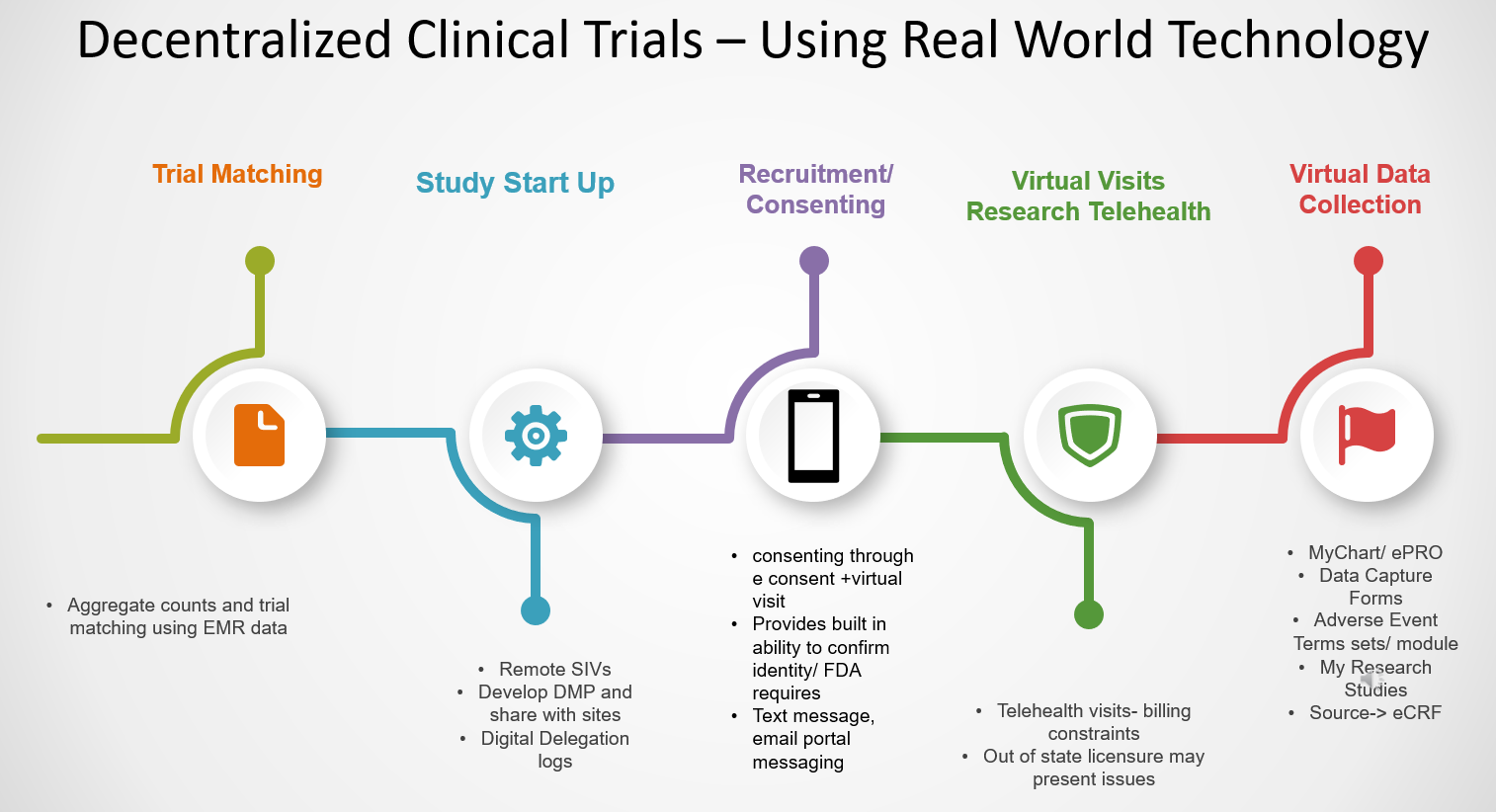
Trial Matching Tools/ Study Start up and Sharing
-
Slicer Dicer
- Slicer Dicer may be used to identify, and message through MyPennMedicine/ MyChart, potential patients who maybe eligible or interested in a trial. It is available for researchers at Penn to use and includes only structured data elements available in the EMR/PennChart.
- Cosmos
- Cosmos data science tools can be used to look at a broad based EMR/Epic based population for epidemiology, observation and public health research. Cosmos can also be used to support decentralized clinical trials. Queries can be generated by a lead site across the network to find counts and matches for potential sites. Messages can then be sent to those sites, via an application called Teleport, to see if the sites want to participate and identify their participants. Beyond site and patient matching, future functions include the ability to build studies, medications, forms, questionnaires, orders sets, etc. and share them from one site across all participating sites.
- TriNetX
- TriNetX is a research cohort identification, data analytics and trial matching tool. It may be used by sponsors to identify counts of patients at sites. That query and trial invite can then be sent from the sponsor to the site for participation. If a site agrees to be part of the trial, the aggregate query can be run locally, for example at Penn, to identify the potentially eligible patients for enrollment.
Transcription and Translation
Decentralized trials can assist team in developing a more regionally and ethnically diverse trial population. This may lead to an increased need for both translation and transcription service on a trial.
- Quantum is Penn Medicine’s preferred translation service tool. This can be used for consents, translating medical records, subject materials, etc. HUP Interpreter Services - Document Translation (upenn.edu)
- Datagain Transcription can be used for research data that needs to be transcribed. Datagain Transcription – High-quality audio and video transcription service (datagainservices.com). For transcription with EMR / patient data there are tools available within the EMR/ PennChart.
Recruitment
-
MyPennMedicine Messaging/ Recruitment for All
- Recruitment through MPM can ow be sent to patients who do not have an active MyChart/ MPM account. This allows research teams to message patients about potential trial opportunities even if they do no have an active MPM account.
- Mobile Apps - Mobile technologies allow research teams to reach participants and conduct parts or all of their study remotely. They may start by supporting secure recruitment and go beyond. Some potential tools at Penn are:
- Way2Health: Link to product’s website HERE
- Social Media – Social media can be a way to connect with potential research patients across the globe. All clinical trial and research study social media ads must be placed on Penn Medicine-Clinical Research pages (Facebook, Instagram). More information and guidelines around using social media in research can be found here.
Consenting
Consenting for clinical research studies can be conducted via electronic signature tools and combined with a virtual visit. Below are a few of the options at Penn for collecting electronic consent.
- REDCap
- Penn Medicine REDCap is an electronic data capture tool that can be used for capturing informed consent documentation electronically for studies that do not require Part 11 compliant signatures. A benefit of REDCap is that all consents are also stored in the database at the patient level as a pdf. More information on consenting in REDCap can be found here.
- DocuSign
- DocuSign is Penn’s part 11 compliant electronic signature tool. It is a great way to consent in FDA regulated trials and is fairly straightforward for research subjects. More information on how to get access, cost and training materials can be found here.
- MyPennMedicine (MPM)
- MPM is Penn Medicine’s, EMR connected patient portal. It provides patients access to scheduling, results and direct communication with their provider. It also has a series of research functions one of which is the ability for patients to consent through MPM. This methods does require that subjects in the study be patients in the Penn EMR/PennChart and that they be active MPM users. More information about consenting in MPM can be found here.
- Advance App
- The Advance platform allows research teams to create consents in a mobile app creation platform, including consent review, and e-signatures. For more information about access, compliance and build materials can be found here.
Virtual Visits
- Telemedicine- Virtual visits can be conducted using telemedicine visits. If the visit will involve clinical and research procedures, then the Clinical Research Connected Health Telemedicine Guidance must be followed and the PennChart applications used. Penn Medicine Teams or University Zoom are also HIPAA compliant and may be used for research only visits.
Data Collection
- Electronic Patient Reported Outcomes (ePRO) – these are electronic systems that allow patients/ subjects to directly report the status of health conditions, complete surveys , communicate with the research.
- MyCap
- MyCap is a participant-facing mobile application for survey data collection and the automated administration of active tasks performed by participants using mobile device sensors. It is a REDCap based tool that can be used via PSOM’s REDCap at Penn for patient reported outcomes that populate into a research REDCap database.
- Advance App
- The Advance application can be used for patient reported outcomes and data collection. At this time it is not directly connected to the EMR or any existing data capture tools. More info can be found here.
- MyPennMedicine (MPM)
- MyPennMedicine (MPM can be used for patients who are active portal users to send questionnaires, messages, forms, etc. for completion during the course of a study. The questionnaires and messages and sent can even be tailored based on the specific research status a patient is in.
- Way to Health
- Way to Health (W2H) is a web-based platform that provides technology infrastructure for sustainable behavior change interventions. The platform's flexible design and focus on automation enable users to deploy tailored solutions for specific patient populations
- MyCap
- Wearable Data Collection - Wearable data are technology tools that can enhance data collection, especially of real world data, in decentralized and centralized clinical trials. It tends to contribute more dynamic and precise patient centric data since it is collected from device directly on the patient without their need to intervene.
- Patients can connect their google fir or Apple Health accounts using an Android or iPhone to the MyChart/MyPennMedicine application. This can send data from wearables directly to the MPM/ EMR.
- Apple Flyer
- Android Flyer - more information coming soon
- Patients can connect their google fir or Apple Health accounts using an Android or iPhone to the MyChart/MyPennMedicine application. This can send data from wearables directly to the MPM/ EMR.
- Electronic Data Capture through APIs – to increase the speed of data collection research teams may be able to take advantage of direction from the Electronic Medical Record (EMR) to their study data capture systems. Some options at Penn for this are :
-
- Clinical Pipe
- Clinical Pipe is middleware used to connect our EMR data elements to a trial data capture system. It may be used for industry or non industry sponsored trials. Currently it can be used with RAVE EDC or Veeva EDC.
- App Orchard Epic APIs
- Epic has some existing APIs as well to support direct EMR data transfer. One of these is a connection between the EMR and REDCap. This can only be used vis the PSOM version of REDCap. To request use of this for a clinical research trial please complete the following link to begin the process, https://redcap.med.upenn.edu/surveys/?s=AY3EENLCANJYT888
- Clinical Pipe
-
Artificial Intelligence Applications
Many studies are looking to use AI/ML tools to see how they compare for subject screening, research recruitment, to develop algorithms or generative AI tools. Penn has developed AI capabilities to assist with this in a research setting. Both of these are HIPAA Compliant tools.
- PennAI Chat: Powered by GPT 3.5 Turbo (GPT 4 coming) from Open AI, PennAI Chat is hosted internally in a HIPAA-compliant environment, which ensures all data and communications are behind our firewalls, logged, and retained securely.
- PennAI Cloud Service: Built on Azure and Databricks within our HIPAA-compliant environment, the PennAI cloud service is a development and delivery environment with open source and commercial large language models available and access to datasets from numerous clinical and operational systems that are updated daily.
More information coming soon on Sidekick and AI features in the EMR.
Medication Administration
More info coming soon
Home Care Visits
More info coming soon
Some Considerations and Challenges with Decentralized Trials
-
Telemedicine– if there is any provision of care during the visit (physical exam, assessment or rash or AEs, etc.) then the provider needs to be licensed in the state in which the telemedicine visit is taking place. They cannot conduct telemedicine visits on patients out of state licensure. This can pose some challenges in discerning clinical vs. research visits as well as with enrollments of patients out of state.
- Part 11 Compliance- With so much electronic documentation, for FDA regulated trials, part 11 compliance becomes even more important. Part 11 standards dictate that the data is validated and the equivalent of any written documentation or wet ink signed documentation. Part 11 validated systems tend to be more labor intensive and have costs associated with them. At Penn the following are considered Part 11 compliant:
- Veeva Site Vault/ Reg Binder and TMF – for trial regulatory documentation
- Velos Clinical Trial Management System – for subject management and data collection
- DocuSign – for consenting
- Veeva Clinical Data Management System – for data collection
- Training Requirements: Any digital medium being used will require both staff and patient training. The patients or subjects need to be fully trained on the tools they are using and have a clear-cut path to ask questions or get help. They also need to confirm their identity using two factor authentication or other approved methods.
- Technology Requirements: DCTs reduce travel and cost barriers for participants but they also increase reliance on technology, and often smart phones. This can serve as a barrier to certain populations and should be considered during trial development. A reasonable number of mixed methods should be considered for trials. Sometimes smart phones or other technology options may be provided to participants by the trial team.