Research
Determine the function of mTORC1/Stat3 signaling in tendon using mouse genetic models
The limited understanding of the regulatory mechanisms underlying tendon cell maturation and fibrovascular scar formation hinders the development of effective treatment modalities for tendon diseases. We have demonstrated that mTORC1 (mechanistic target of rapamycin complex 1) signaling is a critical regulator for postnatal tendon development and constitutive activation of mTORC1 signaling caused fibrovascular scar-like phenotypes in tendons. However, the precise mechanisms by which mTORC1 regulate tendon cell maturation and fibrovascular scar formation are not known. To investigate the function of mTORC1 in tendon cell maturation and fibrovascular scar formation, we analyzed the tendon-specific mTORC1 loss-of-function (Scx-Cre;Raptorfl/fl) and gain-of-function (Scx-Cre;Tscfl/fl) mouse models. To exam the function of mTORC1 in the differentiation of tendon progenitors into the Col1(2.3)-GFP-positive cells, we generated Scx-Cre;Raptorfl/fl ; Col1 (2.3)-GFP (loss-of-function) and Scx-Cre;Tsc1fl/fl ;Col1 (2.3)-GFP (gain-of-function) mouse models. The results from these studies suggested that mTORC1 signaling inhibits early differentiation of tendon progenitors into Col1a1-expressing tenocytes and morphological maturation.
Stat3 is a transcription factor and plays a crucial role in fibrosis and inflammation via the regulation of cell proliferation and ECM organization. Interestingly, previous studies showed that Stat3 can be activated by mTORC1 signaling. To genetically determine Stat3 as a mediator of mTORC1 function in fibrovascular scar formation in tendons, we performed a genetic rescue experiment by generating three types of the tendon-specific deficient mouse: 1) Scx-Cre; Tsc1fl/fl (tendon-specific mTORC1 gain-of-function mouse model), 2) Scx-Cre; Stat3fl/fl (tendon-specific Stat3 knockout mouse model), and 3) Scx-Cre; Tsc1fl/fl; Stat3fl/fl (tendon-specific Tsc1 and Stat3 double knockout mouse model for rescue experiment). Stat3 deletion partly rescued fibrovascular scar-like phenotype such as neovascularization, inflammatory cell infiltration, disorganized collagen organization caused by constitutive activation of mTORC1 signaling. Our genetic data suggest that Stat3 partially mediates mTORC1 function in fibrovascular scar formation. We are currently received R01 grant from NIH for this study.
Investigate regulatory mechanism underlying tendon maturation using scaffold-free 3D tendon constructs
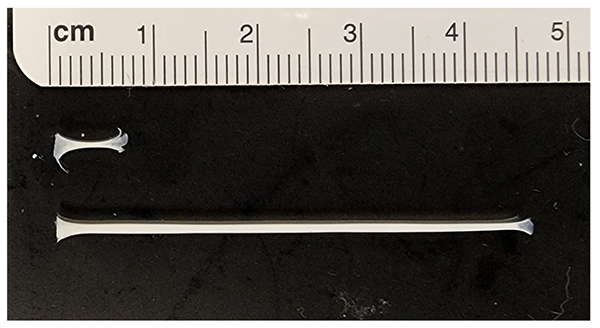
Standard two-dimensional (2D) cell culture has been widely used for in vitro studies to understand molecular mechanisms. However, tenocyte phenotype is not well-maintained in monolayer culture, and it is difficult to study ECM organization and morphological maturation of cells without a 3-dimensional (3D) environment. To overcome this limitation, we developed a scaffold-free, three-dimensional (3D) tendon culture system using mouse tendon cells. The 3D tendon constructs exhibited tissue maturation similar to the postnatal mouse tendon, including decreased cell density, increased thickness, and elongated cells between highly aligned extracellular matrix. The 3D tendon culture system is also feasible for genetic manipulation using adenovirus. Overall, the results suggest that the 3D tendon culture system using mouse tendon cells is a reliable in vitro system to study underlying biological mechanisms that regulate cellular and matrix maturation in postnatal tendon development. we are using this constructs to understand cellular and molecular mechanism regulating tendon maturation.
Publications
- Lawson LY, Migotsky N, Chermside-Scabbo CJ, Shuster JT, Joeng KS, Civitelli R, Silva MJ, Loading-Induced Bone Formation is Mediated by Wnt1 Induction in Osteoblast-Lineage Cells, FASEB J, 2022
- Lee YJ, Park NR, Heo SJ, Mauck RL, Corr DT, Dyment NA, Joeng KS. “Tendon-like cellular and matrix maturation in scaffold-free three-dimensional tendon cell culture using mouse tendon cells”, BioRxiv, 2022, https://doi.org/10.1101/2022.06.08.495368.
- Zhong L, Yao L, Holdreith N, Yu W, Gui T, Miao Z, Elkaim Y, Li M, Gong Y, Pacifici M, Maity A, Busch TM, Joeng KS, Cengel K, Seale P, Tong W, Qin L. “Transient expansion and myofibroblast conversion of adipogenic lineage precursors mediate bone marrow repair after radiation.” JCI Insight. 2022 Apr 8;7(7):e150323. doi: 10.1172/jci.insight.150323.
- Marom R, Burrage LC, Venditti R, Clément A, Blanco-Sánchez B, Jain M, Scott DA, Rosenfeld JA, Sutton VR, Shinawi M, Mirzaa G, DeVile C, Roberts R, Calder AD, Allgrove J, Grafe I, Lanza DG, Li X, Joeng KS, Eyre DR, Westerfield M, De Matteis MA, Lee B. “COPB2 loss of function causes a coatopathy with osteoporosis and developmental delay.” Am J Hum Genet. 2021 Sep 2;108(9):1710-1724. doi: 10.1016/j.ajhg.2021.08.002.
- Turin CG, Joeng KS, Kallish S, Raper A, Asher S, Campeau PM, Khan AN, Al Mukaddam M. “Heterozygous variant in WNT1 gene in two brothers with early onset osteoporosis.” Bone Rep. 2021 Dec;15:101118. doi: 10.1016/j.bonr.2021.101118.
- Park NR, Shetye SS, Bogush I, Keene DR, Tufa S, Hudson DM, Archer M, Qin L, Soslowsky LJ, Dyment NA, Joeng KS, “Reticulocalbin 3 is Involved in Postnatal Tendon Development by Regulating Collagen Fibrillogenesis and Cellular Maturation”, Scientific Reports, 2021, May 25;11(1):10868. doi: 10.1038/s41598-021-90258-8.
- Alhamdi S, Lee YC, Chowdhury S, Byers PH, Gottschalk M, Taft RJ, Joeng KS, Lee BH, Bird LM.Heterozygous WNT1 variant causing a variable bone phenotype. Am J Med Genet A. 2018 Sep 24. doi: 10.1002/ajmg.a.40347.
- Lim J, Munivez E, Jiang MM, Song IW, Gannon F, Keene DR, Schweitzer R, Lee BH, Joeng KS. mTORC1 Signaling is a critical regulator of postnatal tendon development. Scientific Reports, 2017 Dec 7;7(1):17175. doi: 10.1038/s41598-017-17384-0.
- Joeng KS, Lee YC, Chen YQ, Munivez E, Carroll TJ, Ambrose C, Lee BH. The function of osteocyte-derived Wnt1 in osteoblastogenesis and therapeutic potential of Scl-Ab for Wnt1-related osteogenesis imperfecta. The Journal of Clinical Investigation, 2017 Jun 30;127(7):2678-2688. doi: 10.1172/JCI92617. Epub 2017 Jun 19.
- Hudson DM, Weis M, Rai J, Joeng KS, Dimori M, Lee BH, Morello R, Eyre DR. (2017) P3h3-null and Sc65-null Mice Phenocopy the Collagen Lysine Under-hydroxylation and Cross-linking Abnormality of Ehlers-Danlos Syndrome Type VIA. J Biol Chem. 292(9):3877-3887.
- Rajagopal A, Homan EP, Joeng KS, Suzuki M, Bertin T, Cela R, Munivez E, Dawson B, Jiang MM, Gannon F, Crawford S, Lee BH. (2016) Restoration of the serum level of SERPINF1 does not correct the bone phenotype in Serpinf1 null mice. Mol Genet Metab. S1096-7192(15) 30085-8.
- Hudson DM, Joeng KS, Werther R, Rajagopal A, Weis M, Lee BH, Eyre DR. (2015) Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations. J Biol Chem. 290(13): 8613-22.
- Lu L, Harutyunyan K, Jin W, Wu J, Yang T, Chen Y, Joeng KS, Bae Y, Tao J, Dawson BC, Jiang MM, Lee B, Wang LL. (2015) RECQL4 Regulates p53 Function in vivo During Skeletogenesis. J Bone Miner Res. 30(6): 1077-89.
- Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. (2014) Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci U S A. 111(23): 8673-8.
- Joeng KS, Lee YC, Jiang MM, Bertin TK, Chen YC, Abraham AM, Ding H, Bi X, Ambrose C, Lee BH (2014) The Swaying mouse as a model of Osteogenesis Imperfecta caused by WNT1 mutations. Hum Mol Genet. 23(15): 4035-42.
- Joeng KS and Long F (2014) Wnt7b can replace Ihh to induce hypertrophic cartilage vascularization but not osteoblast differentiation during endochondral bone development. Bone Research, 2:14004
- Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Rüegg MA, Hall MN, Ma L, Long F. (2014) WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 10 (1): e1004145.
- Laine CM, Joeng KS (Co-First author), Campeau PM, Kiviranta R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ, Nieminen-Pihala V, Aronen M, Laine T, Kröger H, Cole WG, Lehesjoki AE, Nevarez L, Krakow D, Curry CJR, Cohn D, Gibbs R, Lee BH, Mäkitie O. (2013) WNT1 Mutations in Early-onset osteoporosis and Osteogenesis Imperfecta. N Engl J Med. 368(19): 1809-16
- Joeng KS and Long F. (2013) Constitutive Activation of Hedgehog Signaling Impairs Bone Formation in Postnatal Growing Mice. PLoS One. 8(1): e55134
- Choi SW, Jeong DU, Kim JA, Lee B, Joeng KS, Long F, Kim DW. (2012) Indian Hedgehog signalling triggers Nkx3.2 protein degradation during chondrocyte maturation. Biochem J. 443(3): 789-98.
- Heller E, Hurchla MA, Xiang J, Su X, Chen S, Schneider J, Joeng KS, Vidal M, Goldberg L Deng H, Hornick MC, Prior JL, Piwnica-Worms D, Long F, Cagan R, Weilbaecher KN. (2012)
- Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res. 72(4): 897-907.
- Tu X, Joeng KS (Co-first author), Long F. (2012) Indian hedgehog requires additional effectors besides Runx2 to induce osteoblast differentiation. Dev Biol. 362(1): 76-82.
- Joeng KS, Schumacher C, Zylstra-Diegel CR, Long F, Bart O. Williams. (2011) LRP5 and LRP6 redundantly control skeletal development in the mouse embryo. Dev Biol 359(2): 222-229
- Joeng KS and Long F. (2009) The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilage vascularization. Development, 136(24): 4177-85
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell, 133(2): 340-53.
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F.(2007) Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell, 12(1): 113-27.
- Long F, Joeng KS, Xuan S, Efstratiadis A, McMahon AP. (2006) Independent regulation of skeletal growth by Ihh and IGF signaling. Dev Biol, 298(1): 327-33.
- Joeng KS, Song EJ, Lee KJ, Lee J. (2004) Long lifespan in worms with long telomeric DNA. Nat Genet, 36(6): 607-11
- Yi SY, Joeng KS, Kweon JU, Cho JW, Chung IK, Lee J. (2001) A single-stranded telomere binding protein in the nematode Caenorhabditis elegans. FEBS Lett, 505(2): 301-6.
