Ongoing Research
1. Osteoporosis Treatment and Treatment Strategies
Current Postmenopausal Osteoporosis Treatments
There are two main classes of treatments for osteoporosis, anti-catabolic agents, such as bisphosphonates, and anabolic agents, such as intermittent parathyroid hormone (PTH) treatment. There are many types of anti-catabolic agents, which block osteoclast action to prevent further bone loss. PTH is the only FDA approved anabolic agent for postmenopausal osteoporosis treatment. Our lab has several ongoing studies regarding the benefits and use of PTH as a treatment either alone or in conjunction with other drugs.
Combined PTH and Bisphosphonate Therapy
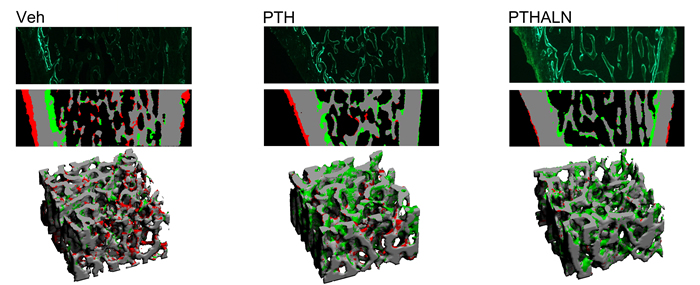
Literature has shown conflicting results regarding combined PTH and biphosphonate therapies. Some groups have found that bisphosphonates blunt the effects of PTH while others reported an additive effect. Our work in this area has shown promising results indicating an additive effect of combined therapy (Fig 1). However, the mechanisms through which this additive effect occurs is unclear. One hypothesis we have proposed is that PTH can promote modeling-based bone formation (Fig 2). Our lab is interested in further identifying the mechanisms through which this additive effect is achieved, and elucidating potential modeling-based formation events by in vivo micro-computed tomography (µCT) and advanced in vivo dynamic imaging techniques.
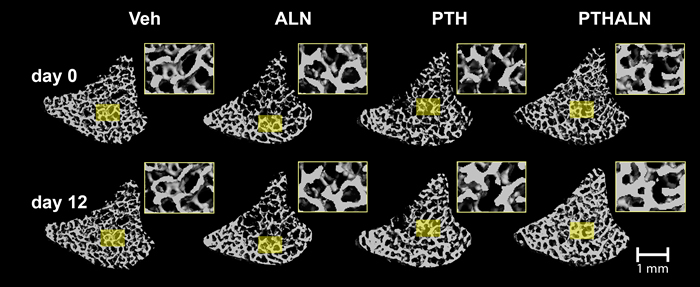
Cyclic and Alternating Anabolic Anti-resorptive Treatment Regimens and Daily PTH Injections
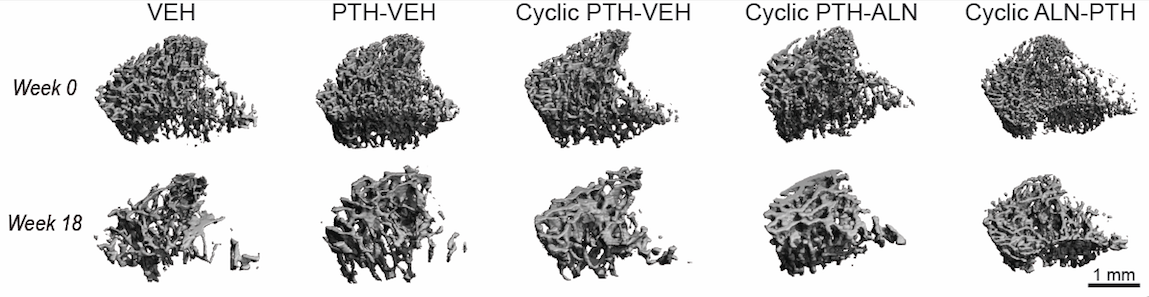
PTH is the first FDA-approved anabolic treatment for postmenopausal osteoporosis. In clinical practice, the recommended treatment duration of PTH is limited to 18-24 months. Despite the potent effects of promoting new bone formation, clinical studies showed a rapid decline in bone mineral density (BMD) upon PTH withdrawal. However, since postmenopausal osteoporosis is a life-long chronic condition, a more sustained and long-term treatment is needed. We are currently investigating the mechanisms behind the adverse effect of PTH withdrawal in an ovariectomized (OVX) rat model of postmenopausal osteoporosis (Fig 3). In order to maximize the efficacy of PTH, we are testing a potential treatment strategy of a cyclic and alternating antiresorptive treatment and a daily PTH injection to rescue PTH withdrawal effects (Fig 4).

2. Dynamics of Modeling- and Remodeling- Based Bone Formation
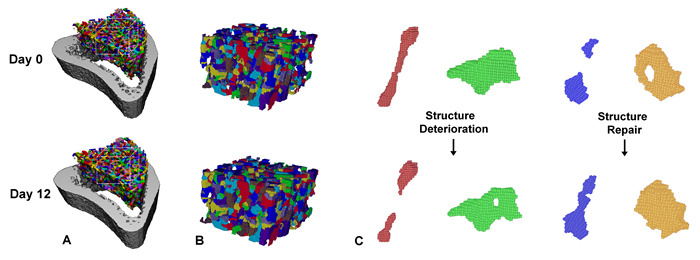
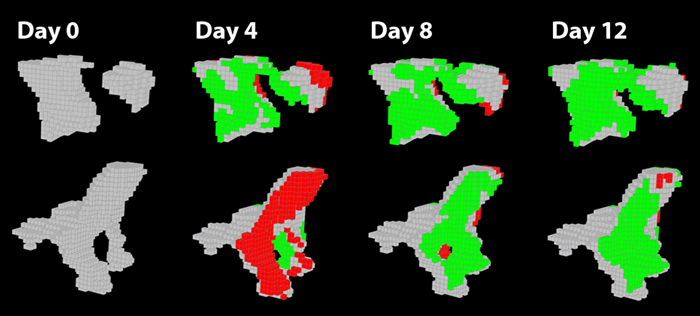
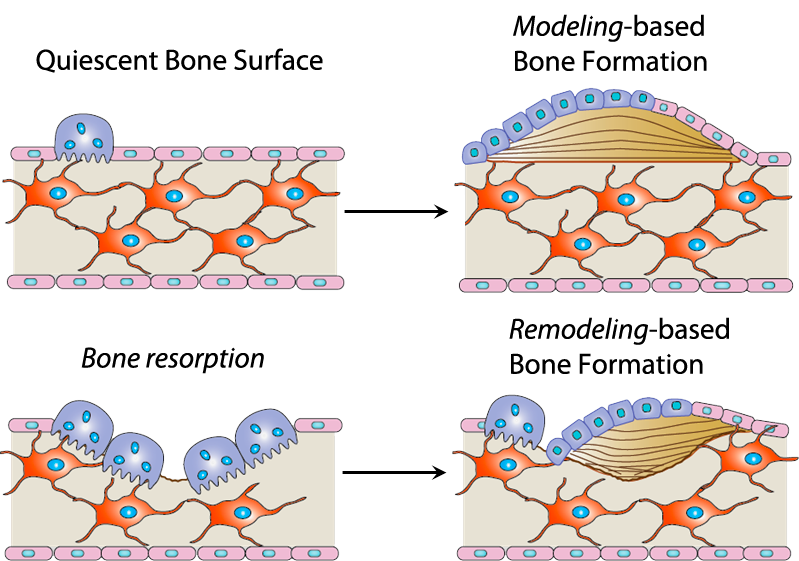
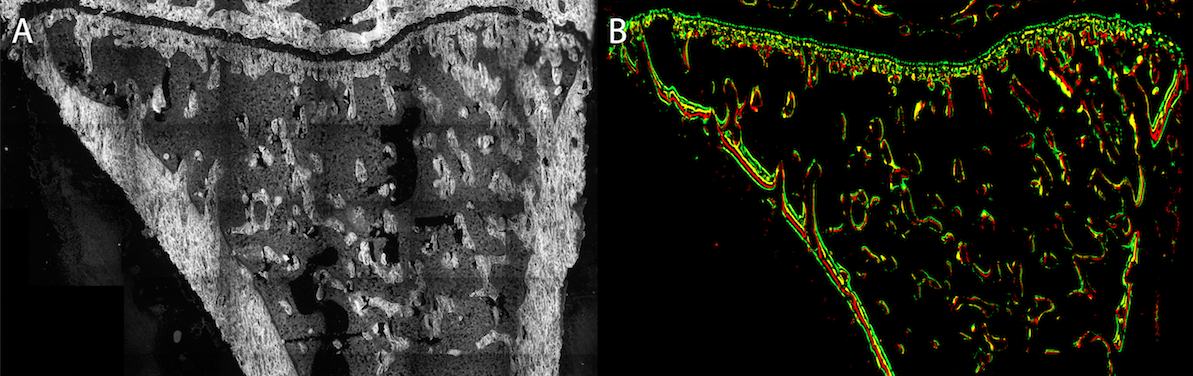
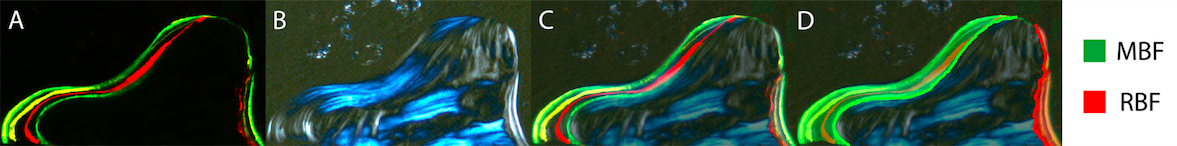
Anabolic agents, like Teriparatide and Abaloparatide, can induce remodeling-based bone formation and modeling-based bone formation. Remodeling-based bone formation (RBF) is when bone formation is linked to prior bone resorption, which helps maintain the mechanical strength of the skeleton through removal of older or fatigued bone. Modeling-based bone formation (MBF) occurs when bone formation is unlinked to prior bone resorption, which contributes to skeletal growth primarily in youth and adolescence (Fig 5). We developed a new technique to identify MBF and RBF. Combined with dynamic histomorphometry, we can observe the relationship between MBF and RBF. We are also interested in the mechanical properties of bone formed through MBF and RBF and what happens to this bone after treatment withdrawal (Fig 6 and 7).
3. Effects of Pregnancy, Lactation, and Weaning on Maternal Bone
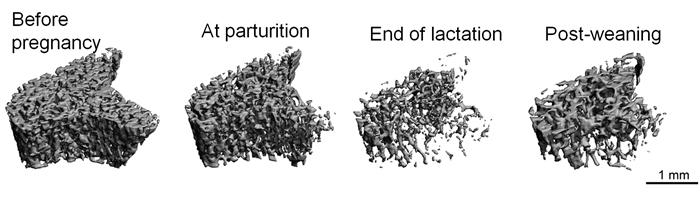
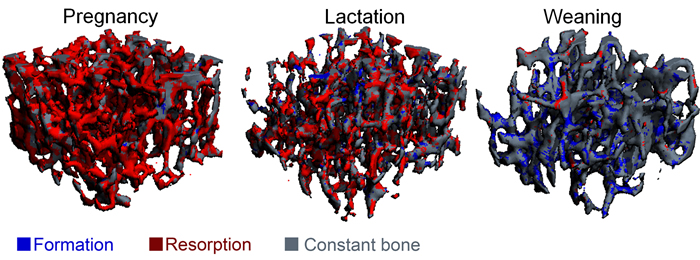
During pregnancy and lactation, the increased calcium demand caused by fetal/infant growth induces substantial changes in maternal calcium metabolism. This affects a variety of physiological processes, including remodeling of the skeleton. In particular, lactation is known to result in dramatic maternal bone loss. This bone loss is partially recovered following weaning; however, even long after weaning, the extent of recovery remains incomplete, and structural and/or mechanical deficits remain (Fig 8 and 9). At the same time, multiple clinical studies indicate that a history of pregnancy and lactation does not have any negative effects on future risk of osteoporosis or fracture. Thus, the long-term effects of pregnancy- and lactation-associated bone loss remain unclear. Using a rat model, combined with advanced in vivo µCT imaging techniques, biological assays, and mechanical and material testing procedures, our lab is working to answer some of these questions. We are currently investigating the ways in which the maternal bone compensates for reproductive bone losses and incomplete recovery post-weaning, through modified bone microstructure, material properties, and altered bone loss patterns post-menopause (Fig 10). We hope that a better understanding of reproductive bone loss and recovery, as well as its effects on post-menopausal bone loss, will lead to improved women's health, both during the reproductive years and post-menopause.
4. Advanced In Vivo Dynamic Imaging and Computational Modeling
Our lab focuses on longitudinal µCT imaging studies in both humans and rodents which allow us to track the same segment of bone over time. In doing this we have developed techniques to measure precise changes in the bone's microarchitecture down to the individual trabecula level (Fig 11 and 12). Our lab is interested in using these imaging and computational tools to gain insight into the complex physiological responses to treatment.