Skeletal Biology and Regeneration
Our group studies the cellular and molecular basis for skeletal development, homeostasis, aging, and disease. Osteoporosis and osteoarthritis are two common skeletal diseases that cause huge economic and social burdens to our society. We utilize cutting edge techniques, including single-cell transcriptome analysis, 3D fluorescent imaging, and genetically modified animal models, to identify mesenchymal stem and progenitor cells at various musculoskeletal sites and investigate the function of progenitors and their descendants under normal and disease conditions. In collaborating with a team of multidisciplinary scientists and clinicians, our ultimate goal is to translate the studies on fundamental mechanisms of skeletal cell function into future clinical applications.
Projects:
Mesenchymal stem and progenitor cells in skeleton
Our group has a long-term interest in identifying skeletal mesenchymal stem and progenitor cells (MSPCs) and understanding mechanisms regulating these progenitors under physiological, pathological and treatment conditions. First discovered in 1970s, bone marrow MSPC is the most well-known one and has been well utilized for tissue regeneration. Our group found its site specificity by demonstrating that endosteal progenitors close to the bone surface are more metabolically active than central progenitors in the midshaft. We also uncovered the critical role of epidermal growth factor receptor (EGFR) in regulating proliferation, differentiation, survival, and migration of bone marrow MPCs, thus contributing to normal bone metabolism and mediating the anabolic action of parathyroid hormone (PTH), an FDA-approved therapy for postmenopausal osteoporosis. In addition to bone marrow, many skeletal sites have residential MSPCs. Our interest in joint development led to a discovery identifying periarticular MPCs responsive for the formation of secondary ossification center during skeletal development. Currently, we are adapting state-of-the-art single cell RNA-sequencing technique to analyze skeletal tissue heterogeneity and delineate stem cell and progenitor subpopulations at various skeletal sites, such as bone marrow, periosteum, joint , and muscle.
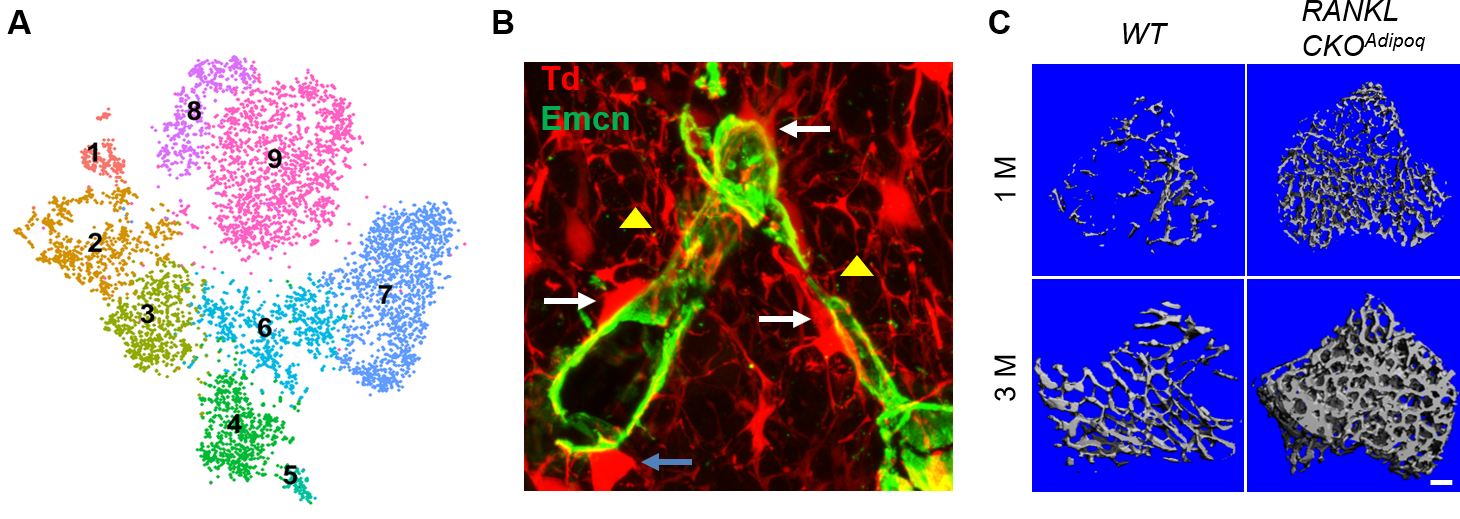
MALP: a new component of bone marrow adipose tissue
Bone marrow mesenchymal lineage cells are a heterogeneous cell population involved in bone homeostasis and diseases such as osteoporosis. By employing large scale single cell transcriptome analysis, we recently computationally defined mesenchymal stem cells, delineated their in vivo bi-lineage differentiation paths, and discovered a new component of marrow adipose tissue that we named marrow adipogenic lineage precursor (MALP). Our studies revealed that MALPs exist abundantly as pericytes and stromal cells forming a ubiquitous 3D network inside the marrow cavity. We also demonstrated that they play critical roles in maintaining marrow vasculature, suppressing bone formation, and promoting bone resorption. The goal of this project is to delineate the molecular mechanisms through which MALPs regulate their bone environment and thus contribute to bone metabolism and skeletal diseases.
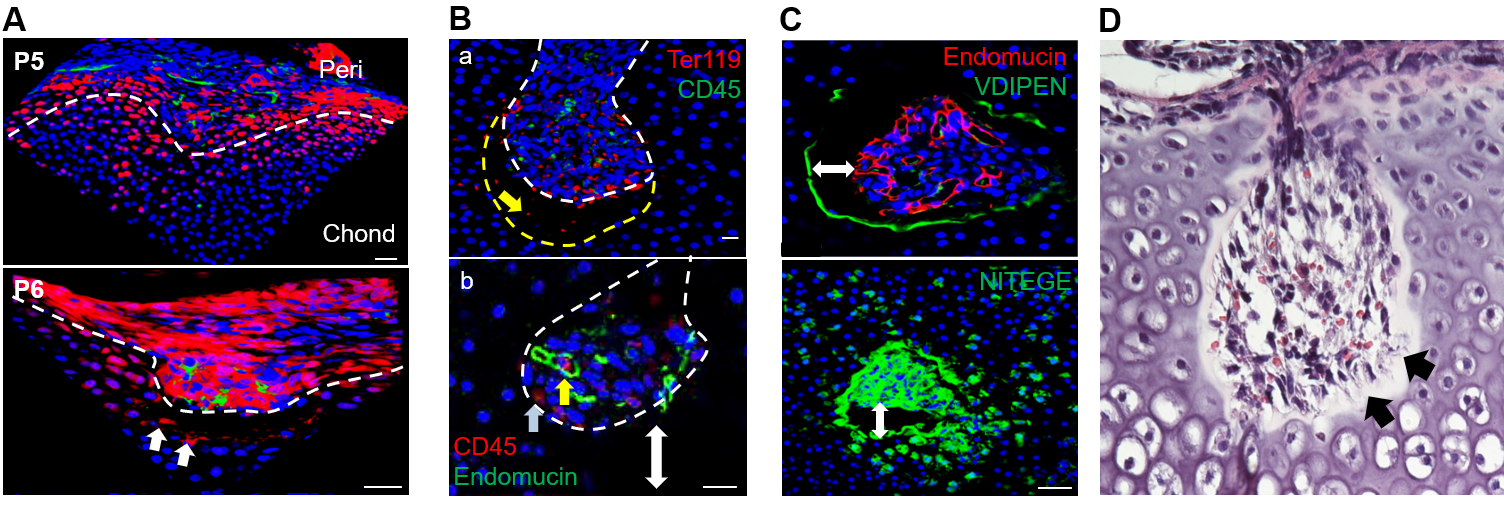
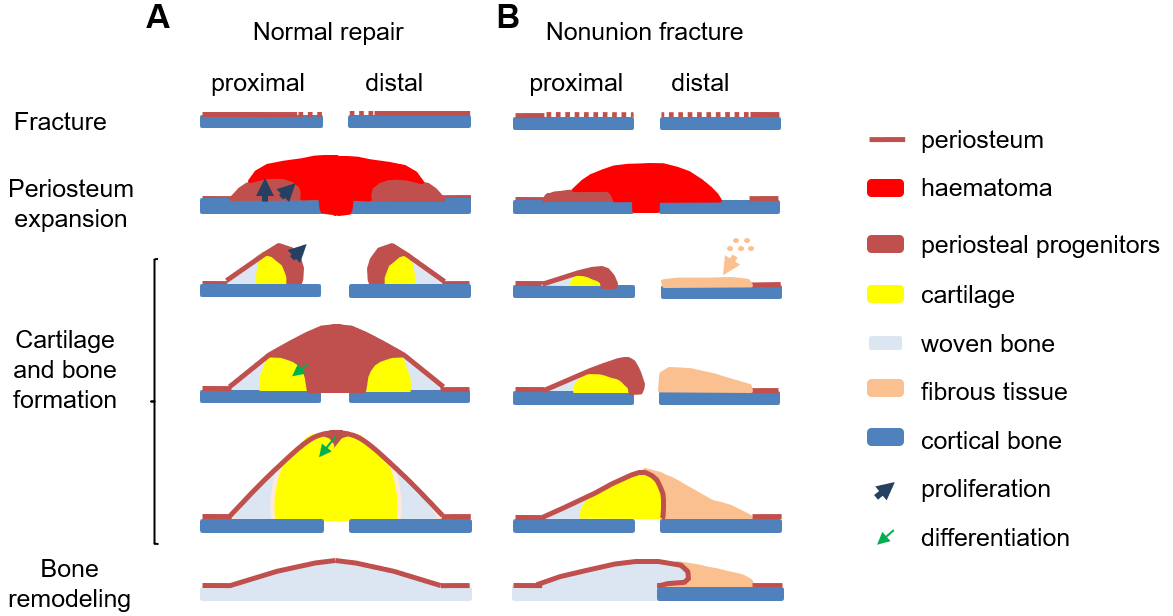
Radiation damage on bone
Radiotherapy is commonly used to eliminate tumors, but there is no approach to prevent or reverse its deleterious effects on tumor-neighboring normal bone tissue. Our group employs a clinically relevant focal radiation model in rodents to study the mechanism by which bone anabolic agents, such as PTH and sclerostin antibody, promote the survival of bone forming osteoblasts and thus attenuate the radiation damage on bone. Moreover, using this radiation model, we investigate atrophic nonunion fractures that are caused by severe damage to the periosteal mesenchymal progenitors and accompanied by an extraskeletal, fibro-cellular response.
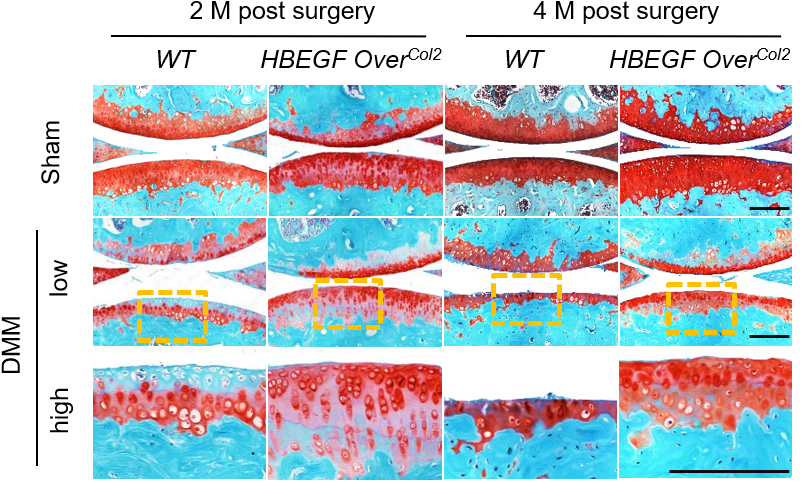
EGFR as a target for osteoarthritis therapy
Osteoarthritis is a widespread joint disease currently with no disease-modifying treatments. My group has pioneered research that identifies the chondrogenic EGFR signaling pathway as an essential regulator of cartilage matrix degradation during growth plate development. By studying the formation of articular cartilage and its osteoarthritis progression, we discovered that EGFR signaling is a novel and important growth factor pathway that maintains MSPCs in the superficial layer, promotes cartilage surface lubrication, and stimulates mechanical strength of articular cartilage. In collaboration with bioengineers, we develop an advanced nanoparticle approach to deliver TGFα, an EGFR ligand, into mouse knee joints as an efficient OA therapy.
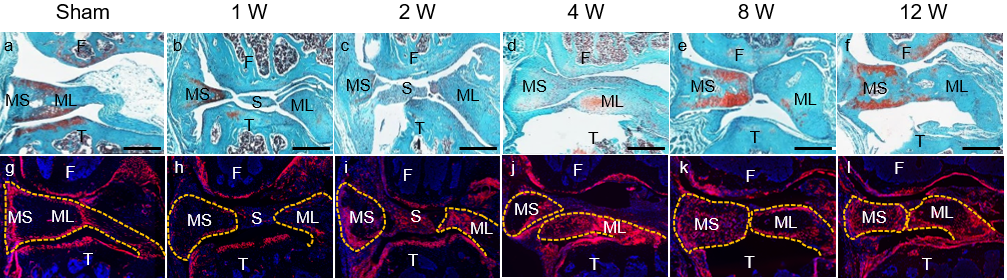
Meniscus injury repair
Meniscal tears are among the most common knee injuries. They are likely to be an important event in the initiation and propagation of osteoarthritis. Various approaches, including stem cell transplantation, have been proposed to repair injured meniscus. However, meniscus-specific progenitors are still largely unknown. Our studies find that Gli1+ cells at the superficial layer of meniscal horns are MSPCs that contribute to meniscus development and injury response. Activation of hedgehog/Gli1 signaling after meniscus injury leads to accelerated meniscus repair and attenuated osteoarthritis progression. Thus, this project is to design a novel bioengineering approach targeting hedgehog/Gli1 pathway in meniscus progenitors to improve healing of tears that are otherwise considered irreparable.