Developmental Mechanobiology and Regeneration
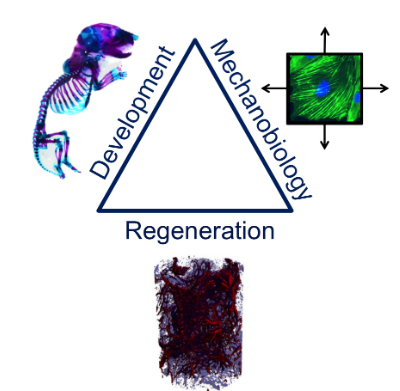
Our laboratory in the Perelman School of Medicine of the University of Pennsylvania is interested in the mechanobiology of development and regeneration. We study how mechanical cues influence morphogenesis, growth, adaptation, and repair, and how these physical cues can be used to engineer functional tissues. We are currently focused on the molecular mechanisms that control cellular mechanosensation during bone and blood vessel development and on creation of tissue engineering strategies that recapitulate these developmental mechanisms for tissue regeneration. Current projects in the lab aim to understand the roles of the transcriptional co-activators YAP and TAZ in regulating cytoskeletal dynamics and cell motility, vasculogenesis, and bone development and repair, as well as engineering vascularized tissues by reverse-engineering the process of development.
Projects:
Transcriptional feedback control of dynamic mechanosensation
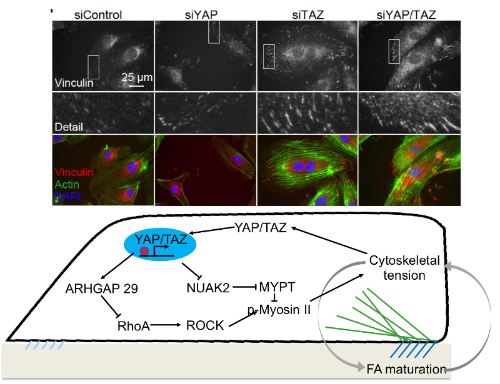
of cytoskeletal/focal adhesion dynamics.
Mechanical cues are converted into biochemical signals through mechanotransduction. Mechanotransduction is critical for a variety of important cell functions, including progenitor cell fate determination. However, the role of mechanotransductive gene expression in regulating mechanosensation itself is poorly understood. We recently showed that transcriptional feedback is required for persistent motility through YAP/TAZ regulation of myosin activation. Here, we ask whether this feedback mechanism also regulates mechanosensation of dynamic changes in matrix rigidity and whether this is mediated by cytoskeletal-nucleoskeletal coupling. This project combines engineered hydrogel systems that enable temporal control of extracellular matrix rigidity with thorough loss-of-function approaches to quantify transcriptional feedback mechanisms in live cells.
This project is supported by the Center for Engineering Mechanobiology at Penn.
Mechanical control of therapeutic vasculogenesis for peripheral ischemia
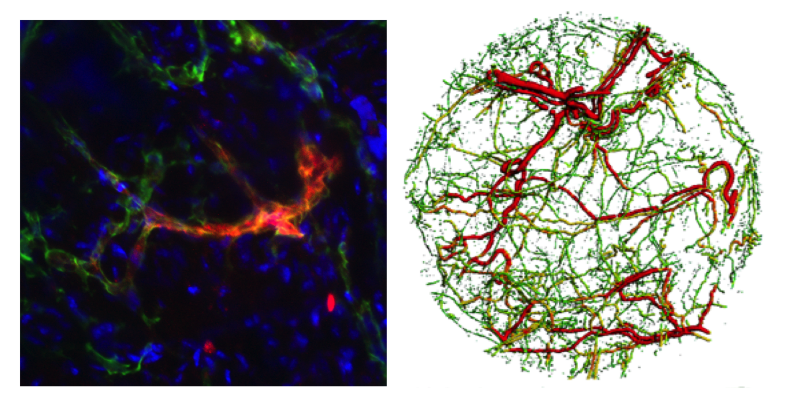
Peripheral artery disease is the third most common cause of cardiovascular morbidity worldwide, present in 20% of the population over the age of 65. If left untreated, it can progress to critical limb ischemia, resulting in necrosis and limb amputation. Vasculogenesis, the process of de novo vessel formation from progenitor cells, may prove an effective therapeutic strategy through delivery of endothelial colony forming cells (ECFCs). Mechanical forces communicated at the cell-matrix interface are known to dramatically influence this process, but the underlying molecular mechanisms are poorly understood, precluding targeted therapeutic strategies. This project aims to develop mechanotransduction-activating devlivery vehicles for vasculogenic endothelial cells.
This project is supported by a Scientist Development Grant (31230034) from the American Heart Association.
Mechanisms of osteocyte mechanotransduction
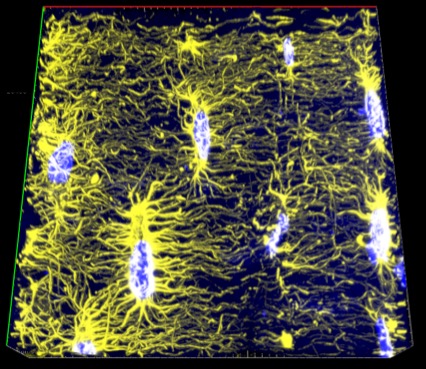
Osteocytes serve as the primary mechanosensors in bone, coordinating bone formation and resorption in response to mechanical demands; however, this coordination is lost in metabolic bone diseases such as osteoporosis, leading to reduced bone mass and increased fracture risk. Our goal is to understand the mechanisms by which osteocytes transduce mechanical signals to regulate skeletal homeostasis and adaptation, which may lead to therapies that can restore the balance in osteoporotic bone. This project aims to define the roles of osteocyte YAP and TAZ in bone homeostasis and adaptation to dynamic loads.
This project is supported by an NIH R21 (AR071559) from NIAMS.
Osteoprogenitor mobilization for bone development and repair
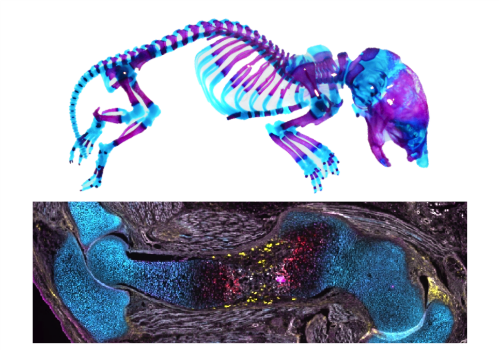
Fluorescently-labeled tissue section of embryonic mouse humerus (bottom).
Large bone defects caused by traumatic injury or tumor resection pose a significant clinical challenge as they cannot not heal without intervention, and current bone grafting therapies are limited. Tissue engineering is a promising alternative, but is often limited by poor recruitment or supply of endogenous progenitor cells. Unlike large defects, bone fractures heal readily by recapitulating the steps of bone development. In fracture repair, mechanical cues, in the form of interfragmentary strains, are essential to determine the pathway by which bone formation occurs, namely direct bone formation through intramembranous ossification or indirect bone formation through endochondral ossification, in which a cartilage template forms first and is later replaced by bone. The mechanisms by which osteoprogenitor cells are mobilized during bone development and how mechanical cues direct fracture repair are poorly understood. We observed combinatorial roles of the mechanosensitive transcriptional co-activators, Yes-associated protein (YAP) and Transcriptional co-activator with PDZ-motif (TAZ) in promoting both bone development and repair. The goal of this project is to define the mechanistic roles of YAP/TAZ in osteoprogenitor cell mobilization during bone development and mechanical load-mediated fracture repair. Accomplishing this goal will provide new insights into developmental bone diseases, identify pathways that could be exploited to enhance healing, and position us to design new tissue engineering strategies that recapitulate the processes of bone development and natural repair for regeneration of large bone defects.
This project is supported by an NIH R01 (AR073809) from NIAMS.
Recapitulating development for bone regeneration
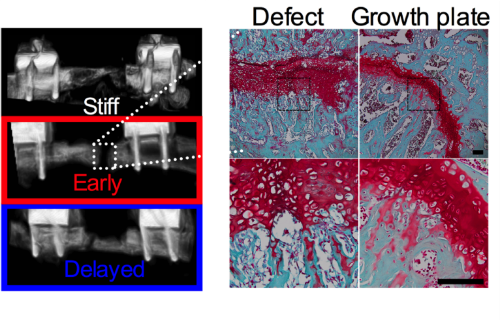
Large bone defects caused by traumatic injury or tumor resection pose a significant clinical challenge as they cannot not heal without intervention, and current bone grafting therapies are limited. Tissue engineering is a promising alternative. Many bone tissue engineering strategies use scaffolds designed to match the structure and properties of mature bone. However, natural fracture healing is most efficient (90-95% success rate) when it recapitulates bone development through endochondral ossification. A tissue engineering therapy that enabled large defects to heal with the same fidelity as natural fracture healing would be transformative. The goal of this project is to understand the mechanisms by which cellular lineage and extracellular matrix influence mechanical regulation of endochondral bone defect regeneration. We will determine the influence of endochondral differentiation state, matrix composition and properties, and YAP/TAZ signaling on mechanical regulation of endochondral ossification using a combination of bioreactor and large bone defect models. These results will identify when and how mechanical stimuli induce endochondral regeneration and may provide insights for development-inspired tissue engineering strategies in other tissues.
This project is supported by an NIH R01 (AR074948) from NIAMS.
