Ongoing Research Projects
Development and Preclinical Evaluation of Bioactive Injectable Therapies for Intervertebral Disc Degeneration and Low Back Pain
Low back pain is the leading cause of disability in the United States, with an estimated socioeconomic cost exceeding $100 billion each year. Intervertebral disc degeneration, a cascade of cellular, compositional and structural changes, is strongly implicated as a cause of low back pain. Current clinical approaches for treating low back pain associated with disc degeneration have limited long term efficacy as they seek only to manage symptoms without restoring native disc structure and mechanical function. There is an overwhelming clinical need for new treatment options, which target not only the symptoms of low back pain but also the underlying causes. Mesenchymal stem cells (MSCs) are an attractive option for cell-based disc regeneration due to their safety, ease of isolation and ability to adopt phenotypes similar to those of disc nucleus pulposus (NP) cells. A major challenge to successful MSC-based disc regeneration, however, is the local cellular microenvironment, which is characterized by limited nutrition, low oxygen and pH, and persistent inflammation. An additional impediment to the clinical application of MSC-based therapies is the lack of preclinical large animal models that effectively recapitulate key characteristics of human disc degeneration. To address this need, our team recently developed a chondroitinase ABC (ChABC)-mediated model of disc degeneration that mimics many of the structural, compositional, inflammatory, and biomechanical characteristics of human disc degeneration. By adjusting the dose of ChABC, we are able to reproducibly control the severity of degeneration. This large animal model, therefore, represents a powerful, clinically-relevant platform for evaluating MSC-based disc therapies.
Key components of this project include:
- Extending the clinical-relevance of this animal model by establishing whether degenerative changes are associated with increased tissue-level expression of inflammatory cytokines and downstream catabolic enzymes, as well as decreased oxygen and nutrient availability.
- Establish an optimized delivery system for neutralizing adverse degenerative disc conditions to improve the survival and function of therapeutic MSCs.
- Preconditioning MSCs prior to implantation using biochemical and biophysical stimulate in order to improve post-implantation survival and function.
- Establishing the in vivo efficacy of a combined MSC and hydrogel therapy for structural and functional regeneration of the intervertebral disc, and alleviation of painful symptoms, in a preclinical animal model
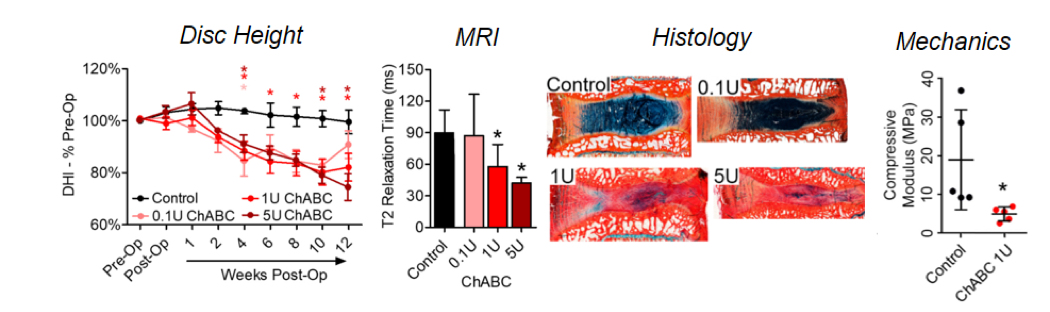
A large animal model that recapitulates the spectrum of human disc degeneration from mild to severe
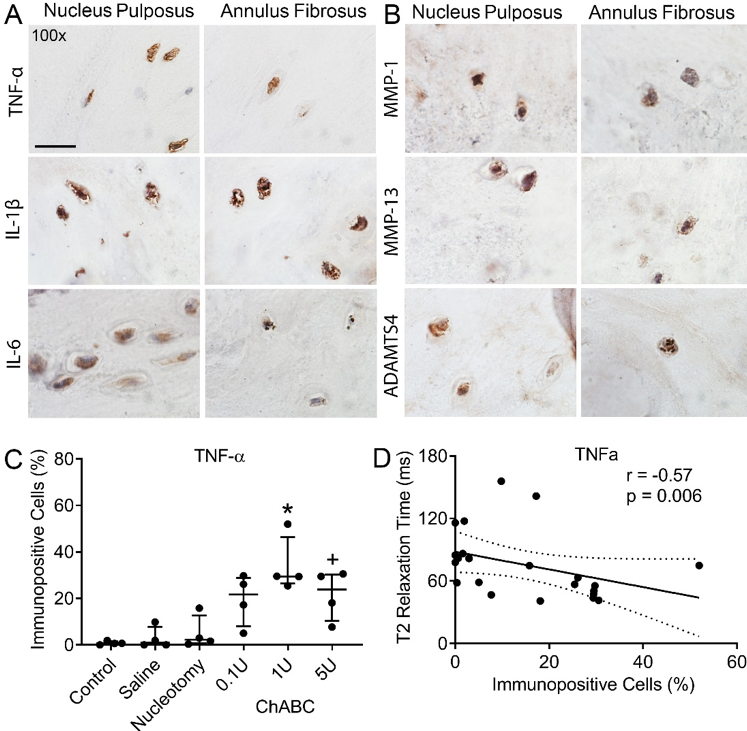
Expression of clinically relevant inflammatory mediators in a large animal model of intervertebral disc degeneration
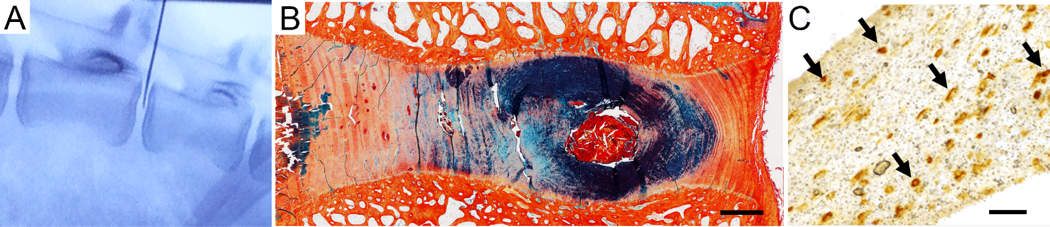
Delivery of injectable hydrogel and mesenchymal stem cells to the intervertebral disc
Publications:
- Gullbrand SE, Schaer TP, Agarwal P, Bendigo JR, Dodge GR, Chen W, Elliott DM, Mauck RL, Malhotra NR, Smith LJ. (2017) Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater. PMID: 28735027
- Gullbrand SE, Malhotra NR, Schaer TP, Zawacki Z, Martin JT, Bendigo JR, Milby AH, Dodge GR, Vresilovic EJ, Elliott DM, Mauck RL, Smith LJ. (2016) A Large Animal Model that Recapitulates the Spectrum of Human Intervertebral Disc Degeneration. Osteoarthritis and Cartilage. PMID:27568573.
- Gorth DG, Lothstein KE, Chiaro JA, Farrell MJ, Dodge GR, Elliott DM, Malhotra NR, Mauck RL, Smith LJ. (2015) Hypoxic Regulation of Functional Extracellular Matrix Elaboration by Nucleus Pulposus Cells in Long Term Agarose Culture. Journal of Orthopaedic Research. 33:747-54. PMID:25640328.
- Showalter BL, Elliott DM, Chen W, Malhotra NR. (2015) Evaluation of an In Situ Gelable and Injectable Hydrogel Treatment to Preserve Human Disc Mechanical Function Undergoing Physiologic Cyclic Loading Followed by Hydrated Recovery. Journal of Biomechanical Engineering.137:081008. PMID:25950273.
- Kim DH, Martin JT, Elliott DM, Smith LJ, Mauck RL. (2015) Phenotypic Stability, Matrix Elaboration, and Functional Maturation of Nucleus Pulposus Cells Encapsulated in Photocrosslinkable Hyaluronic Acid Hydrogels. Acta Biomaterialia. 12:21-9. PMID:25448344.
- Farrell MJ, Shin JI, Smith LJ, Mauck RL. (2015) Functional consequences of glucose and oxygen deprivation on engineered mesenchymal stem cell-based cartilage constructs. Osteoarthritis and Cartilage. 23:134-42. PMID:25241241.
- Gorth DG, Martin JT, Dodge GR, Malhotra NR, Elliott DM, Mauck RL, Smith LJ. (2014) In Vivo Retention and Bioactivity of IL-1ra Microspheres in the Rat Caudal Intervertebral Disc: A Preliminary Investigation.Journal of Experimental Orthopaedics. 1:15. *Highly Accessed
- Smith LJ, Gorth DJ, Showalter BL, Chiaro JA, Beattie EE, Chen W, Elliott DM, Mauck RL, Malhotra NR. (2014) In Vitro Characterization of a Stem Cell-Seeded Triple Interpenetrating Network Hydrogel for Functional Regeneration of the Nucleus Pulposus. Tissue Engineering: Part A. 20:1841-9. PMID:24410394.
- Gorth DG, Mauck RL, Chiaro JA, Mohanraj B, Hebela NM, Dodge GR, Elliott DM, Smith LJ. (2012) IL-1ra Delivered from Poly(Lactic-co-Glycolic Acid) Microspheres Attenuates IL-1β Mediated Degradation of Nucleus Pulposus In Vitro. Arthritis Research and Therapy, 14:R179. PMID:22863285.
- Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM, Mauck RL, Dodge GR, Elliott DM(2011). Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine treatment following long term agarose culture. European Cells and Materials, 22:291-301. PMID:22102324.
Funding Sources:
Department of Veteran’s Affairs, Sharpe Foundation
Updated on 15 Dec 2019
