Current Research Projects
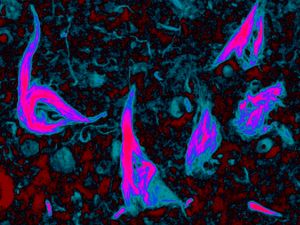 VCP & Vacuolar Tauopathy:
VCP & Vacuolar Tauopathy:
We recently described a new type of hereditary dementia called vacuolar tauopathy. This disease is a subtype of frontotemporal lobar degeneration with tau inclusions (FTLD-tau) where the degeneration is linked to the formation of neurofibrillary tangles that are morphologically and biochemically similar to those seen in Alzheimer's disease. Vacuolar tauopathy is associated with a p.Asp395Gly mutation in VCP which we hypothesize inhibits its ability to disaggregate tau fibrils. We are using protein biochemistry and structural biology methods to better understand how VCP interacts with tau fibrils. Techniques include in vitro recombinant protein biochemistry, human pathologic tissue protein extractions, cyro-electron microscopy, and small molecule screens.
VCP & Multisystem Proteinopathy:
A related question is how different mutations in VCP leads to diverse phenotypes. In contrast with vacuolar tauopathy, other VCP mutations cause a disease called multisystem proteinopathy which manifest either as inclusion body myositis, Paget's disease of bone, or frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP). A fundamental question is how does different mutations of the same gene result in the accumulation of tau protein in vacuolar tauopathy versus TDP-43 protein in multisystem proteinopathy. To address this question, we are using cell culture models, CRISPR-Cas9, various reporter assays, and knock-in mouse models to better understand the cell biology and pathophysiology of VCP.
 C9orf72 and TDP-43:
C9orf72 and TDP-43:
A long standing interest of the laboratory has been to develop a better understanding of the molecular mechanisms that link mutations in C9orf72 and downstream TDP-43 proteinopathy. We have established that epigenetic silencing of C9orf72 appears to be a disease modifier in the setting of frontotemporal degeneration and have developed CRISPR-Cas9 methods to specifically methylate the endogenous genomic locus via homology directed repair. Ongoing studies are examining how cells maintain proper proteostasis in the setting of expressing dipeptide repeat proteins that are encoded by the C9orf72 repeat expansion. We have also pioneered methods to study the molecular consequences of TDP-43 proteinopathy by developing methods to fractionate human brain tissue for transcriptome and genome-wide analyses. We are expanding these studies to better understand the molecular basis for selective vulnerability in FTLD-TDP using single cell and spatial transcriptomics.
Trauma-Related Neurodegeneration:
Finally, there is strong evidence that head trauma is associated with downstream neurodegeneration. However, the full neuropathologic spectrum of TRauma-Related NeuroDegeneration (TReND) has not been fully characterized. We are working with an international consortium of investigators to capture and analyze autopsied tissues with history of trauma, sports and/or military exposure to achieve a more comprehensive understanding of the diverse neuropathologies associated with head trauma.

