Tissue Mechanics, Biomaterials, and Tissue Engineering
R01 EB008722 NIH/NIBIB
Dual PIs: Jason Burdick and Robert Mauck
Title: Engineering Developmental Microenvironments: Cartilage Formation and Maturation
Time Period: 4/13/09-04/20205
|
|
|
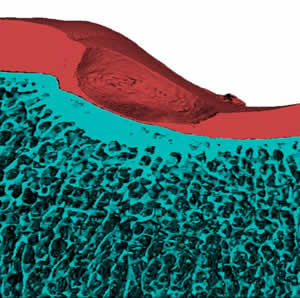
The major goal of this project is to optimize the use of customized hyaluronic acid hydrogels to control in vitro and in vivo cartilage formation using adult human mesenchymal stem cells.
R01 AR056624 NIH/NIAMS
Dual PIs: Robert Mauck and Jason Burdick
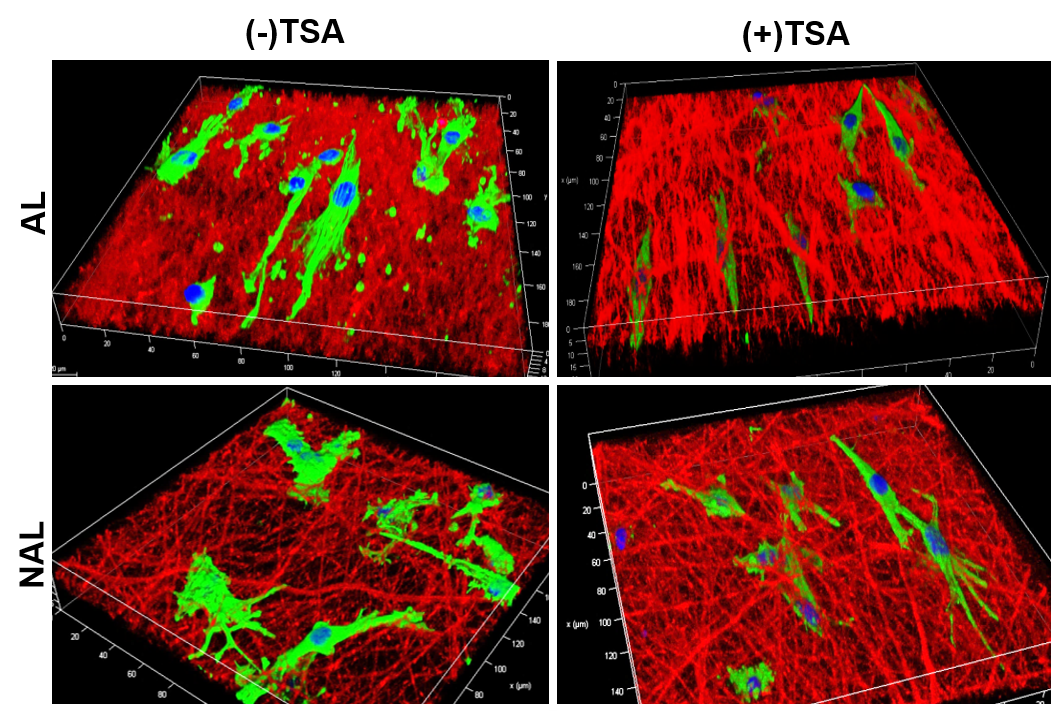
Title: Dynamic Fibrous Scaffolds for Repairing Dense Connective Tissues
Time Period: 7/1/08-11/2024
|
|
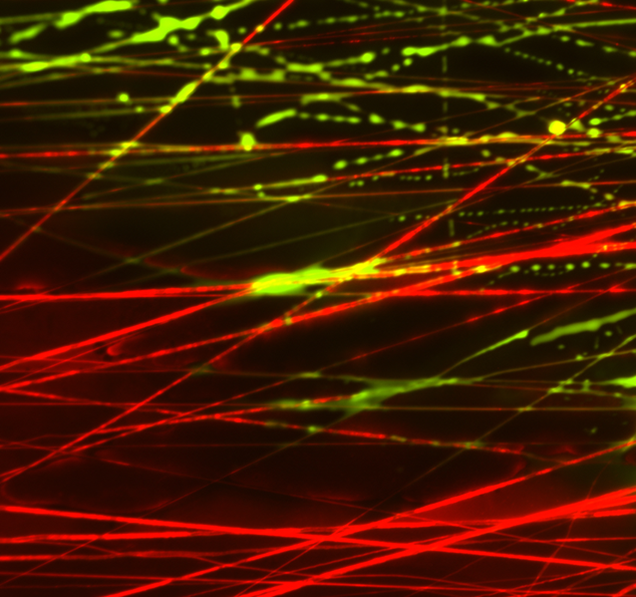
The major goal of this project is to develop novel photo-crosslinkable nanofibrous composites with varying mechanics and degradation profiles to delivery agents that influence cell mechanics so as to expedite the repair of the meniscus and other dense fiber-reinforced tissues.
R01 AR071340 NIH/NIAMS
PIs: George Dodge, Daeyeon Lee, and Robert Mauck
Title: Tunable Mechano-Activated Microcapsules for Therapeutic Delivery
Time Period: 9/21/17-8/31/21
 |
 |
The major goal of this project is to further the development of mechanically activated microcapsules (MAMCs) as a novel controlled drug-delivery system that releases biofactors in response to specific mechanical inputs. In this work, we tune and model mechanical activation through material selection and microcapsule design, with the goal of enabling in vivo delivery in the context of physiologic and clinically relevant loading modalities (e.g., walking, running, therapeutic passive motion during rehabilitation).
R01 AR077435
PIs: Robert Mauck, Lachlan Smith and Neil Malhotra
Title: Neutralizing the degenerate disc microenvironment to enhance the efficacy of therapeutic stem cells
Time Period: 2/2021 – 12/2025
The overall objective of this proposal is to develop a novel biological therapy for disc degeneration that maximizes the survival and anabolic potential of therapeutic stem cells by neutralizing the degenerate disc microenvironment via the sustained delivery of nutrients, anti-inflammatory drugs and buffering agents.
R01 AR079875
PIs: Ling Qin and Robert Mauck
Title: Activation of endogenous progenitors via a nanoparticle-conjugated fibrous system to enhance meniscus repair
Time Period: 02/2023-11/2027
This major goal of this study is to develop a nanoparticle-based drug delivery system to promote meniscus repair by recruiting hedgehog responsive progenitor cell populations.
